1. Introduction
Scientific minds have always expressed a stubborn yearn to master the creation of artificial biological elements in order to emulate the delicate machinery of the human body. The idea of being able to control cell destiny and replace malfunctioning parts of the body with brand new tissues and organs has led to remarkable progress in biological fields such as tissue engineering and regenerative medicine. A promising application of this concept relies on the use of stem cells to originate functional and specialized tissues [
1]. Mesenchymal stem cells (MSCs) constitute a specific subtype of multipotent stem cells, which can differentiate into a variety of cell types and offer the advantage of obtaining pure stem cell populations [
2]. One of the most challenging ambitions in regenerative biomedicine is to restore damaged articular cartilage, as it is one of the most challenging tissue types to heal by virtue of its anatomical and structural complexity. More precisely, the avascular, alymphatic, and aneural nature of the cartilage, combined with the fact that it is characterized by the chondrocyte cell type only, limits its ability to self-repair [
3].
Conventional treatment and modern therapies to treat this singular tissue, ranging from injections to surgical procedures, still suffer, in many instances, from wide variation in clinical outcome, complications, specificity, and effectiveness and a lack of well-grounded long-term reliability [
4]. Joint injection is an easy and minimally invasive procedure for the delivery of MSCs as anti-inflammatory mediators and immune-modulating factors [
5]. Pain relief, improvement in articular function, and regeneration of cartilage following this treatment have been observed in a growing number of studies [
6,
7,
8]. However, the efficacy of this procedure is controversial, since after injection, cells might not survive or remain in situ in the long term [
9,
10]. Satué et al., observed MSCs migrating and engrafting into the damaged cartilage as early as the first day after injection [
11]; in contrast, in another recent study, MSCs were mostly found in the synovium but not in the cartilage surrounding the defect [
12]. Disappearance of the injected cells within the joint may be due to failure in the extracellular matrix (ECM) attachment mechanism [
12]. Although intra-articular injection of MSCs appears to be safe during the short term [
13], further investigations, such as randomized controlled trials, are necessary to explore long-term adverse events and reduce the heterogeneous nature of the studies in terms of design, cell number, exogenous factors, and administration protocols.
Tissue engineering has raised interest as a reasonable approach to manage pathologies like osteoarthritis [
1]. The latter is a degenerative disease characterized by progressive loss of articular cartilage, synovial inflammation, osteophyte formation, and joint space narrowing that lead to overall stiffness, pain, and loss of mobility of the affected joint. The bases of this escalating damage rely on a compromised balance between anabolic and catabolic mechanisms, which can be consequent to several risk factors like ageing, muscle atrophy, metabolic disorders, inflammatory conditions, injuries and overload, or wrong biomechanics of the joint [
14]. This review aims to explore cartilage dynamics and understand what kind of biophysical principles will be beneficial for the engineering-based treatment of degenerative and rheumatic diseases of the joints.
2. Chondrogenesis In Vitro
The essence of regenerative therapy lies in the use of stem cells, bioactive molecules, biomaterials, and their combinations [
15]. Physiological chondrogenesis is regulated by specific cytokines and transcription factors, such as the transforming growth factor beta (TGF-β), fibroblast growth factor (FGF), and insulin-like growth factor 1 (IGF-1) [
16]. As another example, SRY-related high-mobility group box gene 9 (SOX-9) is a transcription factor essential for chondrogenesis processes [
17] and also to avoid dysregulated chondrocyte hypertrophy [
18]. Commonly, MSCs undergo chondrogenic differentiation, recognizable by increasing in specific ECM components, when in the presence of TGF-β. This pathway consists of the proper formation of the receptor complex that requires type I and type II serine/threonine kinase receptors and intracellular effectors. The type I receptor family counts seven members which phosphorylate different small mother against decapentaplegic (SMAD) proteins. Transcriptional activation of chondrogenic genes, like those encoding type II collagen and aggrecan, is mediated by the involvement of the type I receptor 5 (ALK 5) and subsequent phosphorylation of SMAD 2/3. Otherwise, TGF-β could bind ALK 1 and activate the SMAD 1/5/8 pathways, resulting in hypertrophy-related gene expression [
19]. Nevertheless, one of the clinical limits of this kind of chondrogenic differentiation, based on the TGF-β pathway, relies on the risk of incurring undesired and premature hypertrophy entrance of MSCs [
20]. Considering this scenario, a more sophisticated and authentic approach to differentiation is offered by understanding the mechanical forces naturally experienced by chondrogenic progenitors and applying them, even in the absence of exogenous growth factors, to stem cells in order to mimic the same environment within the joint.
3. Influencing the Mechanical Environment
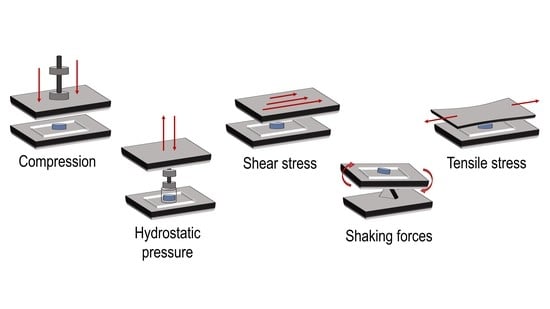
The characteristic structure of the articular cartilage is the result of the dynamic processes that occur within the joint. Chondrocytes experience biomechanical stimuli like compression, shear stress, and hydrostatic pressure [
19]. These forces are perceived as a shifting of currents, electrical fields, or changes in osmolarity and so converted into intracellular signals, influencing mechanisms like transcription, exocytosis, and activity of Na+/K+-ATPase [
21]. Growth factors such as TGF-β, IGF, and bone morphogenetic proteins (BMP)-2,-4,-7 are necessary to stimulate chondrogenic processes and require the presence of calcium ions (Ca
2+) to regulate cell functions, such as the synthesis of extracellular matrix components. Physical stimuli have been associated with the regulation of Ca
2+ entry, primarily through voltage-operated calcium channels (VOCCs), transient receptor potential (TRP) channels, and purinergic receptors [
22]. Furthermore, VOCC inhibitors have shown to reduce cartilage degradation and the progression of osteoarthritis [
23], suggesting their importance in both physiological and pathological milieux. TRP channels, such as TRP vanilloid 4 (TRPV4), which have been linked to upregulation of the SOX9 pathway, or TRPC1, able to guide chondrogenesis in stem cells and regulate the activity of other voltage-dependent ion channels, are also highly involved. Inhibitors for these two receptors, 2-aminoethoxydiphenylborane (2-APB) and Ruthenium Red, have been shown to prevent MSC chondrogenesis induced by pulsed electromagnetic fields (PEMFs) [
24]. The structure of the articular cartilage comprises several layers: First, a thin superficial zone, where the collagen fibers are aligned parallel to the surface and the chondrocytes are numerous and flattened. This layer is in tight contact with the synovial fluid, and it can resist shear stresses. Under the first zone, there is a transitional zone which contains especially proteoglycans (PGs) employed for compressive resistance. Here, the collagen is arranged obliquely. Just below, the deep zone can cushion the compressions and presents collagen fibers organized perpendicular to the surface, high concentrations of proteoglycans, and columns of cells. Lastly, the tide mark separates this last zone from the calcified cartilage [
25] (
Figure 1).
For the sake of simplicity, this tissue could be imagined like a biphasic model: one phase is represented by the interstitial water that permeates the other phase, made up of the solid components of the ECM (collagen, PGs and other proteins). These two phases are extremely interdependent in terms of functionality and biomechanics. Compression is one of the forces experienced by cartilage, which leads to an internal increase of hydrostatic pressure of the aqueous phase. As a result, the water leaks from the ECM towards the capsule, but the structure of the cartilage will remain unaltered thanks to the PG component. More precisely, the effect of uniaxial compression will be compensated by the tensile stiffness generated by the repulsive forces between the negatively charged carboxylic or sulfonic groups of the glycosaminoglycans (GAGs). When the pressure ceases, the water is again attracted inside the interstices. Tissue compressibility under load reaches even a millimeter, but, when the spring back is not able to compensate hard and long compressions, the structure can be damaged [
26]. Shear or rotational stress, which could be defined by the change in thickness with respect to the original height, is another force generated by joint movement and is caused by the tangential friction of synovial fluid on the surface. This movement allows synovial fluid to nourish the cartilage, transport waste materials, and keep the chondrocytes metabolically active with a mechanism of diffusion and fluid convection [
27]. Under this condition, the collagen network is the viscoelastic component of the tissue that exhibits cushioning ability. The collagen concentration is directly proportional to resistance to shifting [
28]. Weight-bearing articular cartilage of the hip and knee daily experience stress amplitudes from 0.5 to 7.7 MPa and average compression of 13% [
29,
30,
31]. Chondrocytes show selective responses to various mechanical stimuli. Indeed, dynamic stresses are able to improve the production of ECM components, while static compressions do not lead to great achievements in tissue engineering constructs. Mechanical stimulation triggers those pathways that culminate in maintaining functional ECM in order to provide substantial physical stability against the stresses to which the cartilage is subjected. It is a feedback cycle in which the mechanical stress influences the production of those components which sustain the stress itself. It is not surprising that, in fact, an unbalanced step in this cycle could pave the way to a pathological mechanism which could, in the end, lead to the onset of osteoarthritic features. If physiological stimulation fails, following, for example, sedentary habits, or it exceeds the ability of the tissue to sustain it, e.g., excessive mechanical loading [
32], the chondrocytes will miss most of the input to produce the new ECM, resulting in unbalanced homeostasis. It is highly recommended to patients who suffer from early osteoarthritis, who are able to conduct physical exercise, to encourage the movement of the diseased articulation in order to stimulate the restoration of the physiological cycle, which may lead to improvements in biochemical disorders. This dynamic environment should be considered in tissue engineering approaches in order to realize as realistic a construct as possible. The challenge proposed is to move from a purely biological view of the natural cell to an engineered one.
Stem cells are studied as structures able to sense and transmit physical stimuli, translating them into biological and mechanical responses, since they have greater mechanical sensitivity than adult cells [
33,
34]. As already mentioned, ion channels are paramount in triggering those signaling pathways which lead to matrix turnover and homeostasis, and an intracellular increase of Ca
2+ levels has been considered as one of the stem cell responses to mechanical load. Sequestration of calcium ions and inhibition of VOCCs and other channels have been shown to attenuate the effects of mechanical stimulation. More specifically, during physical stimulation of MSCs, Ca
2+ is known to be involved in the activation of pivotal transcription factors leading to chondrogenic differentiation [
20]. In the next section, some interesting works on the topic are presented. In some of those, no exogenous growth factors were added during the experiment. Hence, chondrogenic differentiation was achieved exclusively as a result of load applications.
Figure 2 illustrates the different types of mechanical forces applied in these experiments.
4. Practical Applications
To reveal the physiological effect of mechanical stimulation on stem cells, loading machines were designed to be able to apply different types of mechanical forces with different intensity, duration, and frequency.
Cochis et al. [
35] cultivated MSCs in a methylcellulose solution retained within a porous polyurethane matrix to evaluate the suitability of the matrix in supporting mechanically induced chondrogenesis of the cells in the absence of exogenous factors. The composite underwent a combination of compression and shear forces by the use of a bioreactor which applied compressive and rolling movements through a ceramic ball. This ball compressed the scaffold dynamically at 1 Hz, resulting in a strain amplitude of 10–20% of its height, and provided shear stress with oscillations perpendicular to the scaffold axis of ±25° at 1 Hz. After 21 days, the samples were analyzed by reverse transcription polymerase chain reaction (RT-PCR), biochemical, histochemical, and immunofluorescent assays, and the authors concluded that the physical stimulation led to the activation of TGF-β and to accumulation in the surrounding matrix of glycosaminoglycans and type II collagen, confirming the chondrogenesis process.
Another study on the involvement of mechanical forces in the destiny of adipose-derived mesenchymal stem cells (ADSCs) by Zhang et al. [
16] highlights the role of dynamic compression in combination with exogenous SOX-9 on chondrogenesis. ADSCs were seeded in 3D porous polylactic-co-glycolic acid (PLGA) scaffolds. Gradual, meaning with a unique structure at each level, and uniform scaffolds were subjected, with the use of a bioreactor, to sequential uniaxial compressions of 5–10% strain amplitude and frequency of 0.1 Hz. The authors analyzed the morphology of the ADSCs and the ECM deposition within the scaffolds by scanning electron microscopy (SEM), noticing that ECM accumulation by ADSCs, on gradual scaffolds and in the presence of SOX-9, was higher than that in uniform scaffolds or in gradual scaffolds without SOX-9. RT-PCR analysis also showed the highest expressions of Proteoglycan 4 (PRG4), Parathyroid hormone-related protein (PTHrP), type II collagen, aggrecan, SOX-9, and Hypoxia-inducible factor 1-alpha (HIF-1α) in the group of SOX-9 gradual scaffolds.
To better understand the single contributions of compression and shear forces in chondrogenic induction, Schatti et al. [
27] analyzed these two stimuli, both alone and in combination. MSCs were seeded onto polyurethane scaffolds and underwent either compression at 1 Hz, in a strain amplitude of 10–20%, or oscillation of ±25° at 1 Hz, or a combination of both loads. Oscillation was imposed through the shifting of a ball perpendicularly to the scaffold axis. Superimposed compression was applied along the axis of the scaffold as well. The results suggested that stimulation by combined strains, instead of the application a single stimulus alone, is the best way to assure a chondrogenic phenotype in the absence of exogenous growth factors. The authors gave evidence of significant upregulation, in comparison to the control group, of the chondrogenesis markers (type II collagen, Aggrecan (AGG), Cartilage oligomeric matrix protein (COMP), SOX-9) only in the samples loaded with both compression and shear. Also, this last group was the only one in which type II collagen immunostaining was detected and that seemed to maintain a constant release of GAG in medium.
Cheng et al. [
36] developed a novel construct made up by platelet-rich fibrin (PRF) membrane, which functions as a growth-factor-rich scaffold for bone-marrow-derived stem cells (BMSCs), for transplantation in cartilage defects. Flexibility of the neo-formed cartilage and differentiation of the stem cells were achieved through stimulation by hydrostatic pressure, in order to achieve boundaryless tissue consistency between the formed neocartilage and the damaged host cartilage in the temporomandibular joint (TMJ). TMJ offers an interesting environment for evaluating the integration of a cartilage construct within the damaged surface and to compare the behavioral differences of fibrocartilage in comparison to hyaline cartilage of the knee joint. In TMJ, the control of applied loads is difficult because of the impossibility to immobilize this joint, so the adaptation and responses of the construct to biophysical stimuli are more consistent and are extremely suitable to be studied as a model for cartilage regeneration approaches [
37]. The hydrostatic pressure device, in this experiment, applied compression ranging from 90 to 150 kPa, revealing that proliferation and chondrogenic markers of the BMSC/PRF constructs were highest during the first days and gradually decreased at 6 days, suggesting that BMSCs could have limited chondrogenic capacity in relation to decreased growth factor release from the PRF. The authors concluded that pressure is an indispensable stimulus in order to promote cell proliferation, tissue regeneration, and repair mechanisms and to obtain a physiologic hierarchical and polar arrangement of the neoformed tissue.
4.1. Influence of Cell Distribution
Gardner et al. [
38] simulated the multiaxial mechanical loads that characterize the articular joint and observed the results derived from applying these forces on fibrin–poly(ester-urethane) scaffolds seeded with MSCs. The constructs were divided into three groups, represented in
Figure 3: in the first group, the cells were evenly dispersed throughout the scaffold (Uniform); in the second group, the cells were asymmetrically disseminated within the matrix, forming a thin layer on the surface (Asymmetric); and in the third group, the cells were allowed to adhere only to the upper face of the scaffold (Surface Only). These different distributions were investigated in terms of matrix deposition in response to mechanical stress. The protocol employed 20 cycles of 10% compression, achieved by the raising and lowering of a ball onto the scaffold. The rotation on the ball generated shear friction of ±25° at 1 Hz. Histological and immunohistochemical analysis showed that there was an increase in glycosaminoglycans and type II collagen levels in the Asymmetric group in comparison to the other two groups. Besides this, the cells in the Surface Only group produced a small amount of matrix, suggesting a hypertrophic-like phenotype. In conclusion, the pattern of cell distribution within the scaffold is a critical parameter to take into consideration, and matrix deposition could be enhanced by considering the anisotropic properties of the materials.
Cell distribution can also be controlled by physiological mobilization of the cells, from their niches to different areas of the scaffold, employing biomechanical stimulation. Long-lasting regeneration of articular cartilage after surgical techniques like autologous chondrocyte implantation (ACI), mosaicplasty, and microfracture can be hampered by failure to attract progenitor cells, leading to the formation of fibrocartilage. In vitro loading compression provided by a bioreactor, such as 10% strain at 0.3 Hz frequency, applied intermittently for 24 hours, was shown to induce the mobilization of MSCs from a reservoir to an alginate scaffold located above it [
39]. This experiment aimed to provide a model of a cartilage defect in the tibial plateau in order to evaluate the possible effect of biomechanical loading on cell recruitment from the subchondral bone. The mobilization of the stem cells from the reservoir required the supporting effect of laminin-521 (LN-521), as it is a basement membrane protein which exerts a pivotal role in cell adhesion and migration mechanisms. The processes involved in antigravity migration within the scaffold are still the object of study and could imply extracellular signals between the cells and also physical fluidic movements induced by the stimulation of the bioreactor.
4.2. Osteogenic Involvement
Another recent study by Carrol et al., presented the role of mechanical stimulation in MSC osteogenic differentiation [
40]. This work explains about the ability of cyclic tensile strain (CTS) to regulate the initiation of MSC differentiation and, more specifically, their involvement in the endochondral pathway. MSCs embedded in fibrin hydrogels experienced uniaxial tensile deformation in a novel bioreactor system. The authors found out that CTS, in the absence of differentiation factors, can enhance the expression of tenogenic and osteogenic markers. The lack of evident chondrogenesis suggests that CTS could take part in directly initiating intramembranous ossification. When in the presence of chondrogenic growth factor (TGF-β3), instead, CTS induced increased proteoglycan and collagen production and enhanced upregulation of the markers of endochondral ossification (Bone morphogenetic protein 2 (BMP2), Runt-related transcription factor 2 (RUNX2), (Alkaline phosphatase) ALP, Osteopontin (OPN), Collagen Type X Alpha 1 Chain (COL10A1)). The authors concluded that CTS is an inducer of both endochondral and intramembranous ossification of stem cells, depending on the environment.
Endochondral ossification-based engineering techniques are promising strategies to provide regeneration of large defects. Hybrids of bone and cartilage tissue from induced pluripotent stem cells (iPSCs) have been achieved by the combined use of osteogenic and chondrogenic media in addition to mechanical stimulation, by means of shaking forces [
41]. iPSCs offer the advantage of self-organizing in culture medium into cell aggregates, known as embryoid bodies (EB), without the support of scaffolds, providing a model to examine mechanisms of tissue differentiation and organ development. In this experiment, the aggregates, when maintained in osteogenic medium culture, underwent osteogenic induction, reaching mineralization; when, instead, the aggregates were set first in osteogenic and later in chondrogenic medium, they were able to originate both tissues by expressing osteogenic and chondrogenic marker genes. The percentages of bone and cartilage composition seem to be subject to variations in culture periods, medium components, and shaking frequency, allowing the generation of easily manipulated osteochondral organoids.
4.3. Co-Cultures
Co-cultures of articular chondrocytes (ACs) and MSCs have been proposed to overcome problems associated with the dedifferentiation of chondrocytes during in vitro expansion or to the tendency of MSCs to acquire hypertrophic features [
42,
43]. In co-cultures, the milieu created by the chondrocytes can, in fact, stimulate stem cell differentiation, which, in turn, acts as an enhancer of phenotype stability and the proliferation of chondrocytes by stimulating cell–cell adhesion mechanisms and by secreting several paracrine factors like growth factors and cytokines. Proper mechanical stress, in the form of cyclic sinusoidal dynamic tensile mechanical stimulation, can stimulate the co-culture by improving the deposition of ECM (GAGs, type II collagen) and the expression of regulatory factors (TGFβ, SOX9) and promoting the exchange of molecules between MSCs and chondrocytes [
44].
Co-cultures offer the advantage of overcoming the tissue engineering challenge of maintaining a sufficient number of functional chondrocytes which will provide structural reliability with the right amount of ECM. Scaffolds embedded with ADSCs, subjected to cyclic compression in bioreactors, could benefit from the presence of chondrocytes, since they are able to release paracrine factors (i.e., TGF-β1 and IGF-1), guiding stem cells towards chondrogenic differentiation. This approach has been used to reduce the use of exogenous growth factors, which can instead be synthesized by a proper number of chondrocytes during in vitro expansion [
35]. Even though similar production of type II collagen and GAGs was observed in single and co-cultures, the latter were shown to be able to suppress the expression of Col I, Col X, and Tumor necrosis factor alpha (TNF-α) markers [
45].
4.4. Computational Approach
The spreading use of bioreactors has offered the possibility to observe, in a feasible and controlled way, the process of differentiation of stem cells under mechanical stresses, leading to expanded understanding of the mechanisms underpinning the relationship between physical stimulation and biological responses. The outcome of these kinds of stimulation is strongly dependent on the design of the machine, the type of mechanical forces, their intensity and frequency, and the times of stimulation, not to mention the variables related to the scaffolds and the cells. Therefore, the results coming from different studies lead to high heterogeneity and difficulties in providing a uniform consensus about the best protocol to induce processes of differentiation. For this reason, computational models can help, since they have been suggested and used to further investigate tissue engineering strategies. These approaches could be employed to characterize the mechanical stresses imposed by the bioreactors, refine scaffold geometry, and analyze physiobiological dynamics and cell behavior [
46]. In silico experiments complement in vitro and in vivo analysis, addressing some complex questions which can be difficult to answer through more traditional approaches. Theoretical assumptions, simplifications in the conceptual framework, and the need to operate consistent validations could limit their use. Koh et al. [
47] investigated the mechanisms of cartilage regeneration in osteochondral defects by using 3D medical imaging of the knee joint and analyzing the mechano-regulation processes underlying MSC differentiation. Two computational finite element (FE) models were employed to investigate the effects of physical stresses on cell regenerative mechanisms, as well as physiological processes like mitosis and cell death. The aim of the models was to provide predictions of the influence of different loading conditions on the whole system. The results indicated that simulation of a stance-phase gait cycle performed according to the ISO14,243-1 standard [
48] induces more consistent cartilage regeneration than simulation of a vertical loading. This is because when the vertical loading was simulated, it was predicted that endochondral ossification would sustain bone development and that hydrostatic pressure would induce the formation of fibrocartilage.
5. Conclusions
Designing tissue engineering strategies for articular cartilage requires a thorough control of stem cell fate. The latter can be achieved through a more traditional approach using growth factors or, as suggested by the studies discussed above, by applying precise extrinsic mechanical loads, able to mimic the environment within the joint. Therefore, mechanical loading has been proposed as an alternative strategy to induce MSC chondrogenesis without the use of exogenous factors. Depending on the stimulus applied, promotion of specific tissue-related elements can be achieved. It can be concluded that shear stress and hydrostatic pressure, which exert their maximum effect on the superficial zone of the tissue, can increase type II collagen synthesis. Superimposed compression is, instead, the leading promoter of glycosaminoglycan production, since these components are directly involved in structural maintenance following perpendicular stresses. As a future prospective, functionalized bioengineering needs to take into account that de novo tissues should not lack the excellent organization of the cartilage that is critical for their role. A more in-depth study of the effects of different forces imposed on MSC cultures could represent a possible key to creating the original cartilage disposition with parallel collagen fibers in the superficial zone and perpendicular alignment in the thicker layer. Another aspect that should be investigated is the exact biomolecular mechanism by which the cells respond positively to friction and pressure. Tribology offers paramount principles to follow in order to understand and truly benefit the regenerative sciences.