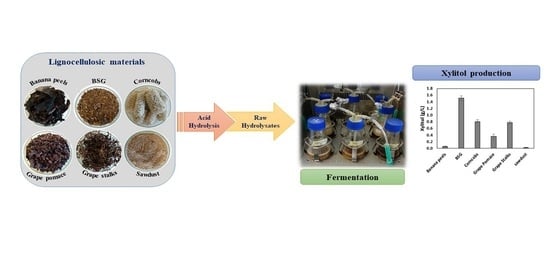
Biovalorization of Lignocellulosic Materials for Xylitol Production by the Yeast Komagataella pastoris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Characterization of the Materials
2.3. Preparation of Hydrolysates
2.4. Preparation of BSG Hydrolysates
2.4.1. Concentration of the BSG Hydrolysates
2.4.2. Detoxification Treatment
2.5. Characterization of Hydrolysates
2.6. Yeast Cultivation Experiments
2.6.1. Yeast Strain and Medium
2.6.2. Inocula Preparation
2.6.3. Shake Flask Experiments
2.6.4. Analytical Techniques
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Raw Materials
3.2. Characterization of the Raw Hydrolysates
3.2.1. Sugar Composition and Hydrolysis Efficiency
3.2.2. Content of Hydrolysis Degradation Products
3.2.3. Phenolic Compounds Content
3.3. Cultivation of K. pastoris on Raw Hydrolysates
3.4. Optimization of K. pastoris Cultivation on BSG Hydrolysate
3.4.1. Detoxification of BSG Hydrolysate
3.4.2. Cultivation of K. pastoris on Detoxified BSG Hydrolysate
4. Techno-Economic Analysis (TEA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. Xylitol: A review on bioproduction, application, health benefits, and related safety issues. Crit. Rev. Food Sci. Nutr. 2015, 55, 1514–1528. [Google Scholar] [CrossRef]
- Barbieri, G.; Barone, C.; Bhagat, A.; Caruso, G.; Conley, R.; Parisi, S. Sweet compounds in foods: Sugar alcohols. In The Influence of Chemistry on New Foods and Traditional Products; Springer: Berlin/Heidelberg, Germany, 2014; pp. 51–59. [Google Scholar]
- Arcaño, Y.D.; García, O.D.V.; Mandelli, D.; Carvalho, W.A.; Pontes, L.A.M. Xylitol: A review on the progress and challenges of its production by chemical route. Catal. Today 2020, 344, 2–14. [Google Scholar] [CrossRef]
- Dasgupta, D.; Bandhu, S.; Adhikari, K.; Ghosh, D. Challenges and prospects of xylitol production with whole cell bio-catalysis: A review. Microbiol. Res. 2017, 197, 9–21. [Google Scholar] [CrossRef]
- Mejia, E.; Pearlman, M. Natural Alternative Sweeteners and Diabetes Management. Curr. Diabetes Rep. 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, C.; Kumar, C.D.; Joseph, J. Xylitol in preventing dental caries: A systematic review and meta-analyses. J. Nat. Sci. Biol. Med. 2017, 8, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, E.; Mazaheri, R.; Tahmourespour, A. Effect of probiotic yogurt and xylitol containing chewing gums on salivary S Mutants Count. J. Clin. Pediatr. Dent. 2017, 41, 257–263. [Google Scholar] [CrossRef]
- Piekara, A.; Krzywonos, M.; Szymańska, A. Sweetening agents and sweeteners in dietary supplements for children-analysis of the polish market. Nutrients 2020, 12, 2387. [Google Scholar] [CrossRef]
- Uebanso, T.; Kano, S.; Yoshimoto, A.; Naito, C.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of consuming xylitol on gut microbiota and lipid metabolism in mice. Nutrients 2017, 9, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldes, A.B.; Vecino, X.; Cruz, J.M. Nutraceuticals and Food Additives. In Current Developments in Biotechnology and Bioengineering: Food and Beverages Industry, 1st ed.; Pandey, A., Du, G., Sanromán, M.Á., Soccol, C.R., Dussap, C.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 143–164. [Google Scholar]
- Wetzel, S.; Duchesne, L.C.; Laporte, M.F. Nutraceuticals from the forest. In Bioproducts from Canada’s Forests: New Partnerships in the Bioeconomy; Springer: Dordrecht, The Netherlands, 2006; pp. 113–130. [Google Scholar]
- Cai, L.; Feng, J.; Regenstein, J.; Lv, Y.; Li, J. Confectionery gels: Effects of low calorie sweeteners on the rheological properties and microstructure of fish gelatin. Food Hydrocoll. 2017, 67, 157–165. [Google Scholar] [CrossRef]
- Ahuja, K.; Mamtani, K. Global Market Insights, Xylitol Market Size by Application (Chewing Gum, Confectionary, Food, Personal Care, Pharmaceuticals, Nutraceuticals), Downstream Application Potential (Xylaric Acid, Ethylene Glycol, Propylene Glycol), Industry Analysis Report, Regional Outlook, Industry Outlook Report, Regional Analysis, Application Potential, Price Trends, Competitive Market Share & Forecast, 2020–2026. Available online: https://www.gminsights.com/industry-analysis/xylitol-market (accessed on 13 April 2021).
- Hernández-Pérez, A.F.; de Arruda, P.V.; Sene, L.; da Silva, S.S.; Kumar Chandel, A.; de Almeida Felipe, M.D.G. Xylitol bioproduction: State-of-the-art, industrial paradigm shift, and opportunities for integrated biorefineries. Crit. Rev. Biotechnol. 2019, 39, 924–943. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, T.L.; da Silva, I.J., Jr.; de Macedo, G.R.; Rocha, M.V.P. Biotechnological production of xylitol from lignocellulosic wastes: A review. Process. Biochem. 2014, 49, 1779–1789. [Google Scholar] [CrossRef]
- Rao, L.V.; Goli, J.K.; Gentela, J.; Koti, S. Bioconversion of lignocellulosic biomass to xylitol: An overview. Bioresour. Technol. 2016, 213, 299–310. [Google Scholar]
- Li, M.; Meng, X.; Diao, E.; Du, F. Xylitol production by Candida tropicalis from corn cob hemicellulose hydrolysate in a two-stage fed-batch fermentation process. J. Chem. Technol. Biotechnol. 2012, 87, 387–392. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Chade, M.; Mereles, E.B.; Bengoechea, D.I.; Brizuela, J.G.; Felissia, F.E.; Area, M.C. Strategies of detoxification and fermentation for biotechnological production of xylitol from sugarcane bagasse. Ind. Crop. Prod. 2016, 91, 161–169. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Acid hydrolysis and fermentation of brewer’s spent grain to produce xylitol. J. Sci. Food Agric. 2005, 85, 2453–2460. [Google Scholar] [CrossRef]
- Silva, C.J.S.M.; Roberto, I.C. Improvement of xylitol production by Candida guilliermondii FTI 20037 previously adapted to rice straw hemicellulosic hydrolysate. Lett. Appl. Microbiol. 2001, 32, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Dharmaraja, J.; Arvindnarayan, S.; Shoban, S.; Bakonyi, P.; Saratale, G.D.; Nemestóthy, N.; Bélafi–Bakó, K.; Yoon, J.J.; Kim, S.H. A comprehensive review on thermochemical, biological, biochemical and hybrid conversion methods of bio-derived lignocellulosic molecules into renewable fuels. Fuel 2019, 251, 352–367. [Google Scholar] [CrossRef]
- Shrotri, A.; Kobayashi, H.; Fukuoka, A. Catalytic conversion of structural carbohydrates and lignin to chemicals. Adv. Catal. 2017, 60, 59–123. [Google Scholar]
- Chandel, A.K.; Da Silva, S.S.; Singh, O.V. Detoxification of lignocellulose hydrolysates: Biochemical and metabolic engineering toward white biotechnology. BioEnergy Res. 2013, 6, 388–401. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef]
- Buhner, J.; Agblevor, F.A. Effect of detoxification of dilute-acid corn fiber hydrolysate on xylitol production. Appl. Biochem. Biotechnol. 2004, 119, 13–30. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Santos, J.C.; Roberto, I.C. Effect of pH and activated charcoal adsorption on hemicellulosic hydrolysate detoxification for xylitol production. J. Chem. Technol. Biotechnol. 2004, 79, 590–596. [Google Scholar] [CrossRef]
- Silva, C.J.; Mussatto, S.I.; Roberto, I.C. Study of xylitol production by Candida guilliermondii on a bench bioreactor. J. Food Eng. 2006, 75, 115–119. [Google Scholar] [CrossRef]
- Rao, R.S.; Jyothi, C.P.; Prakasham, R.S.; Sarma, P.N.; Rao, L.V. Xylitol production from corn fiber and sugarcane bagasse hydrolysates by Candida tropicalis. Bioresour. Technol. 2006, 97, 1974–1978. [Google Scholar] [CrossRef] [PubMed]
- Tochampa, W.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Srinophakun, P.; Bakker, H.H.; Chisti, Y. A model of xylitol production by the yeast Candida mogii. Bioprocess Biosyst. Eng. 2005, 28, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lv, J.; Wang, H.; Wang, B.; Li, Z.; Deng, Z. Genetically engineered Pichia pastoris yeast for conversion of glucose to xylitol by a single-fermentation process. Appl. Microbiol. Biotechnol. 2014, 98, 3539–3552. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Kenealy, W.R.; Jeffries, T.W. Xylitol production from DEO hydrolysate of corn stover by Pichia stipitis YS-30. J. Ind. Microbiol. Biotechnol. 2011, 38, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Verma, S.; Bhatia, R.; Tiwary, B.N. Risks and precautions of genetically modified organisms. ISRN Ecol. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Araújo, D.; Freitas, F.; Sevrin, C.; Grandfils, C.; Reis, M.A.M. Co-production of chitin-glucan complex and xylitol by Komagataella pastoris using glucose and xylose mixtures as carbon source. Carbohydr. Polym. 2017, 166, 24–30. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P. Converting nitrogen into protein beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, D.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; NREL LAP: Golden, CO, USA, 2008; pp. 1–6. [Google Scholar]
- Rehman, S.; Nadeem, M.; Ahmad, F.; Mushtaq, Z. Biotecnological production of xylitol from banana peel and its impact on physicochemical properties of rusks. J. Agric. Sci. Technol. 2013, 15, 747–756. [Google Scholar]
- Carvalheiro, F.; Esteves, M.P.; Parajó, J.C.; Pereira, H.; Gırio, F.M. Production of oligosaccharides by autohydrolysis of brewery’s spent grain. Bioresour. Technol. 2004, 91, 93–100. [Google Scholar] [CrossRef]
- Rivera, P.; Moldes, A.B.; Torrado, A.M.; Domínguez, J.M. Lactic acid and biosurfactants production from hydrolyzed distilled grape marc. Process Biochem. 2007, 42, 1010–1020. [Google Scholar] [CrossRef]
- Salgado, J.M.; Rodríguez, N.; Cortés, S.; Domínguez, J.M. Effect of nutrient supplementation of crude or detoxified concentrated distilled grape marc hemicellulosic hydrolysates on the xylitol production by Debaryomyces hansenii. Prep. Biochem. Biotechnol. 2012, 42, 1–14. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Rivas, S.; Moldes, A.B.; Domínguez, J.M. Simultaneous lactic acid and xylitol production from vine trimming wastes. J. Sci. Food Agric. 2007, 87, 1603–1612. [Google Scholar] [CrossRef]
- Rafiqul, I.S.M.; Sakinah, A.M. Kinetic studies on acid hydrolysis of Meranti wood sawdust for xylose production. Chem. Eng. Sci. 2012, 71, 431–437. [Google Scholar] [CrossRef]
- Kamal, S.M.M.; Mohamad, N.L.; Abdullah, A.G.L.; Abdullah, N. Detoxification of sago trunk hydrolysate using activated charcoal for xylitol production. Procedia Food Sci. 2011, 1, 908–913. [Google Scholar] [CrossRef] [Green Version]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Farinha, I.; Araújo, D.; Freitas, F. Optimization of medium composition for production of chitin-glucan complex and mannose-containing polysaccharides by the yeast Komagataella pastoris. J. Biotechnol. 2019, 303, 30–36. [Google Scholar] [CrossRef]
- Barakat, A.; de Vries, H.; Rouau, X. Dry fractionation process as an important step in current and future lignocellulose biorefineries: A review. Bioresour. Technol. 2013, 134, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Zhang, J.A.; Ling, H.Z.; Ping, W.X.; Huang, W.; Ge, J.P.; Xu, J.M. Optimization of pH and acetic acid concentration for bioconversion of hemicellulose from corncobs to xylitol by Candida tropicalis. Biochem. Eng. J. 2009, 43, 203–207. [Google Scholar] [CrossRef]
- Ping, Y.; Ling, H.Z.; Song, G.; Ge, J.P. Xylitol production from non-detoxified corncob hemicellulose acid hydrolysate by Candida tropicalis. Biochem. Eng. J. 2013, 75, 86–91. [Google Scholar] [CrossRef]
- Rafiqul, I.S.M.; Sakinah, A.M. Processes for the production of xylitol—A review. Food Rev. Int. 2013, 29, 127–156. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Medeiros, R.; Gírio, F.M. Optimization of brewery’s spent grain dilute-acid hydrolysis for the production of pentose-rich culture media. In Proceedings of the Twenty-Fifth Symposium on Biotechnology for Fuels and Chemicals, Breckenridge, CO, USA, 4–7 May 2003; Humana Press: Totowa, NJ, USA, 2004; pp. 1059–1072. [Google Scholar]
- Duarte, L.C.; Carvalheiro, F.; Lopes, S.; Marques, S.; Parajo, J.C.; Gírio, F.M. Comparison of two posthydrolysis processes of brewery’s spent grain autohydrolysis liquor to produce a pentose-containing culture medium. In Proceedings of the Twenty-Fifth Symposium on Biotechnology for Fuels and Chemicals, Breckenridge, CO, USA, 4–7 May 2003; Humana Press: Totowa, NJ, USA, 2004; pp. 1041–1058. [Google Scholar]
- Villarreal, M.L.M.; Prata, A.M.R.; Felipe, M.G.A.; Silva, J.A.E. Detoxification procedures of eucalyptus hemicellulose hydrolysate for xylitol production by Candida guilliermondii. Enzyme Microb. Technol. 2006, 40, 17–24. [Google Scholar] [CrossRef]
- Mattos, G.N.; Tonon, R.V.; Furtado, A.A.; Cabral, L.M. Grape by-product extracts against microbial proliferation and lipid oxidation: A review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Mussato, S.I.; Dragone, G.; Roberto, I.C. Influence of the toxic compounds present in brewer’s spent grain hemicellulosic hydrolysate on xylose-to-xylitol bioconversion by Candida guilliermondii. Process Biochem. 2005, 40, 3801–3806. [Google Scholar] [CrossRef]
- Kumdam, H.; Murthy, S.N.; Gummadi, S.N. Arabitol production by microbial fermentation-biosynthesis and future applications. Int. J. Sci. Appl. Res. 2014, 1, 1–12. [Google Scholar]
- Chagas, B.; Farinha, I.; Galinha, C.F.; Freitas, F.; Reis, M.A.M. Chitin–glucan complex production by Komagataella (Pichia) pastoris: Impact of cultivation pH and temperature on polymer content and composition. New Biotechnol. 2014, 31, 468–474. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Lopes, S.; Parajó, J.C.; Pereira, H.; Gírio, F.M. Evaluation of the detoxification of brewery’s spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Process Biochem. 2005, 40, 1215–1223. [Google Scholar] [CrossRef]
- Almeida, J.R.; Bertilsson, M.; Hahn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Carbon fluxes of xylose-consuming Saccharomyces cerevisiae strains are affected differently by NADH and NADPH usage in HMF reduction. Appl. Microbiol. Biotechnol. 2009, 84, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Kinetic behavior of Candida guilliermondii yeast during xylitol production from brewer’s spent grain hemicellulosic hydrolysate. Biotechnol. Prog. 2005, 21, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Swart, L.J.; Petersen, A.M.; Bedzo, O.K.; Görgens, J.F. Techno-economic analysis of the valorization of brewers spent grains: Production of xylitol and xylo-oligosaccharides. J. Chem. Technol. Biotechnol. 2021, 96, 1632–1644. [Google Scholar] [CrossRef]


 ) and hydrolysis efficiencies (
) and hydrolysis efficiencies (  ) of the different raw materials: (A) banana peels, (B) brewer’s spent grain (BSG), (C) corncobs, (D) grape pomace, (E) grape stalks, and (F) sawdust. GluR, XylR, and AraR represents the percentage of cellulose, xylan, and arabinan converted into glucose, xylose, and arabinose, respectively.
) of the different raw materials: (A) banana peels, (B) brewer’s spent grain (BSG), (C) corncobs, (D) grape pomace, (E) grape stalks, and (F) sawdust. GluR, XylR, and AraR represents the percentage of cellulose, xylan, and arabinan converted into glucose, xylose, and arabinose, respectively.
 ) and hydrolysis efficiencies (
) and hydrolysis efficiencies (  ) of the different raw materials: (A) banana peels, (B) brewer’s spent grain (BSG), (C) corncobs, (D) grape pomace, (E) grape stalks, and (F) sawdust. GluR, XylR, and AraR represents the percentage of cellulose, xylan, and arabinan converted into glucose, xylose, and arabinose, respectively.
) of the different raw materials: (A) banana peels, (B) brewer’s spent grain (BSG), (C) corncobs, (D) grape pomace, (E) grape stalks, and (F) sawdust. GluR, XylR, and AraR represents the percentage of cellulose, xylan, and arabinan converted into glucose, xylose, and arabinose, respectively.

| Raw Material | Concentration of H2SO4 (% w/w) | Liquid to Solid Ratio (% w/w) | Time of Hydrolysis (min) | Reference |
|---|---|---|---|---|
| Banana peels | 1 | 10 | 40 | [36] |
| BSG | 3 | 8 | 20 | [37] |
| Corncobs | 6 | 10 | 40 | [17] |
| Grape pomace | 3.3 | 8 | 30 | [38,39] |
| Grape stalks | 3 | 8 | 20 | [40] |
| Sawdust | 6 | 8 | 20 | [41] |
| Composition | Banana Peels | BSG | Corncobs | Grape Pomace | Grape Stalks | Sawdust |
|---|---|---|---|---|---|---|
| Moisture (%) | 89.7 ± 0.4 | 72.1 ± 0.8 | 8.9 ± 0.9 | 58.9 ± 0.6 | 9.7 ± 0.4 | 9.1 ± 0.5 |
| Ash content (%) a | 13.4 ± 1.7 | 1.3 ± 0.5 | 4.1 ± 0.5 | 8.7 ± 0.8 | 5.4 ± 0.7 | 0.5 ± 1.1 |
| Cellulose (%) a | 21.1 ± 1.3 | 19.0 ± 1.7 | 28.4 ± 0.5 | 12.2 ± 0.4 | 17.6 ± 2.2 | 42.8 ± 1.1 |
| Hemicellulose (%) a | 7.3 ± 0.5 | 17.6 ± 1.2 | 34.1 ± 1.2 | 7.3 ± 0.4 | 7.8 ± 1.0 | 18.0 ± 0.6 |
| Xylans (%) a | 4.8 ± 0.4 | 10.7 ± 1.0 | 30.2 ± 0.9 | 5.9 ± 0.4 | 3.9 ± 0.5 | 17.5 ± 0.6 |
| Arabinans (%) a | 2.5 ± 0.3 | 6.9 ± 0.7 | 3.9 ± 0.8 | 1.4 ± 0.2 | 3.9 ± 0.9 | 0.5 ± 0.1 |
| Total lignin (%) a | 14.1 ± 0.5 | 16.3 ± 0.6 | 17.1 ± 2.4 | 18.1 ± 0.7 | 18.6 ± 0.9 | 17.9 ± 0.5 |
| ASL (%) a | 6.8 ± 0.5 | 11.3 ± 0.2 | 6.3 ± 2.3 | 3.9 ± 0.7 | 7.1 ± 1.3 | 9.6 ± 0.6 |
| AIL (%) a | 7.3 ± 0.1 | 5.0 ± 0.2 | 10.8 ± 2.4 | 14.2 ± 0.2 | 11.5 ± 0.8 | 8.3 ± 0.7 |
| Protein (%) a | 6.6 ± 0.3 | 27.5 ± 0.5 | 3.4 ± 0.4 | 9.8 ± 0.6 | 9.2 ± 0.2 | 0.5 ± 0.1 |
| Raw Hydrolysate | Banana Peels | BSG | Corncobs | Grape Pomace | Grape Stalks | Sawdust |
|---|---|---|---|---|---|---|
| Initial glucose (g/L) | 8.2 ± 0.6 | 6.4 ± 2.3 | 2.3 ± 0.4 | 1.7 ± 0.5 | 8.0 ± 0.7 | 1.7 ± 0.2 |
| Glucose consumed (g/L) | 8.2 ± 1.5 | 4.9 ± 0.9 | 0.7 ± 0.1 | 1.7 ± 0.3 | 6.7 ± 1.2 | 0.1 ± 0.0 |
| Initial xylose (g/L) | 1.2 ± 1.1 | 8.9 ± 2.6 | 8.2 ± 1.5 | 3.1 ± 1.3 | 5.5 ± 1.1 | 10.8 ± 1.9 |
| Xylose consumed (g/L) | 1.0 ± 0.4 | 2.3 ± 0.9 | 2.2 ± 0.8 | 1.7 ± 0.6 | 3.1 ± 1.2 | 0.5 ± 0.2 |
| Initial arabinose (g/L) | 1.4 ± 0.3 | 6.2 ± 2.1 | 0.9 ± 0.2 | 0.9 ± 0.3 | 1.9 ± 0.7 | 0.4 ± 0.1 |
| Arabinose consumed (g/L) | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.1 ± 0.0 |
| CDW (g/L) | 15.18 ± 0.33 | 5.70 ± 0.94 | 13.70 ± 0.15 | 7.08 ± 0.17 | 14.58 ± 0.19 | 0.92 ± 0.09 |
| Xylitol (g/L) | 0.06 ± 0.01 | 1.51 ± 0.07 | 0.81 ± 0.05 | 0.36 ± 0.07 | 0.78 ± 0.04 | 0.02 ± 0.01 |
| Arabitol (g/L) | 0.02 ± 0.01 | 0.90 ± 0.09 | 0.21 ± 0.03 | 0.21 ± 0.02 | 0.34 ± 0.04 | 0.07 ± 0.01 |
| Type of Hydrolysate | Glucose (g/L) | Xylose (g/L) | Arabinose (g/L) | Furfural (g/L) | HMF (g/L) | Acetic Acid (g/L) |
|---|---|---|---|---|---|---|
| Initial | 6.44 ± 1.18 | 8.85 ± 3.23 | 6.19 ± 1.78 | 0.18 ± 0.03 | 0.12 ± 0.02 | 0.78 ± 0.06 |
| Raw (pH adjusted) | 6.44 ± 1.19 | 8.13 ± 2.96 | 5.72 ± 1.65 | 0.15 ± 0.02 | 0.15 ± 0.02 | 0.76 ± 0.06 |
| Concentrated | 11.71 ± 2.16 | 15.22 ± 5.55 | 10.30 ± 2.96 | 0.23 ± 0.04 | 0.30 ± 0.05 | 1.14 ± 0.08 |
| Detoxified | 9.57 ± 1.76 | 13.00 ± 4.74 | 8.85 ± 2.55 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.72 ± 0.05 |
| Type of BSG Hydrolysate | Concentrated | Detoxified |
|---|---|---|
| Initial glucose (g/L) | 11.90 ± 0.19 | 11.68 ± 2.10 |
| Glucose consumed (g/L) | 11.90 ± 0.19 | 11.43 ± 2.06 |
| Initial xylose (g/L) | 14.18 ± 1.04 | 15.65 ± 2.65 |
| Xylose consumed (g/L) | 5.89 ± 0.43 | 7.13 ± 1.21 |
| Initial arabinose (g/L) | 9.87 ± 0.43 | 9.71 ± 0.86 |
| Arabinose consumed (g/L) | 3.98 ± 0.17 | 1.37 ± 0.12 |
| CDW (g/L) | 13.73 ± 0.25 | 20.26 ± 0.25 |
| Xylitol (g/L) | 1.56 ± 0.07 | 3.97 ± 0.10 |
| Yxyl/S (gxylitol/gxylose) | 0.26 ± 0.09 | 0.56 ± 0.17 |
| Arabitol (g/L) | 0.43 ± 0.05 | 0.82 ± 0.05 |
| Yarab/S (garabitol/garabinose) | 0.11 ± 0.09 | 0.60 ± 0.11 |
| Microorganism | Type of Hydrolysate | Additional Treatment | Xylitol (g/L) | Yxyl/s (gxylitol/gxylose) | References |
|---|---|---|---|---|---|
| K. pastoris DSM 70877 | Concentrated (2×) | - | 1.56 ± 0.07 | 0.26 ± 0.09 | This study |
| Activated charcoal detoxification | 3.97 ± 0.10 | 0.56 ± 0.17 | |||
| D. hansenii CCMI 941 | Concentrated (4×) | Activated charcoal detoxification | - | 0.50 | [57] |
| C. guilliermondii FTI 20037 | Raw | - | - | 0.65 | [59] |
| Raw | - | 10.76 | 0.70 | [19] | |
| Raw | Sugars’ supplementation | 62.3 | 0.79 | [54] | |
| Concentrated (4×) | Activated charcoal detoxification | - | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, D.; Costa, T.; Freitas, F. Biovalorization of Lignocellulosic Materials for Xylitol Production by the Yeast Komagataella pastoris. Appl. Sci. 2021, 11, 5516. https://doi.org/10.3390/app11125516
Araújo D, Costa T, Freitas F. Biovalorization of Lignocellulosic Materials for Xylitol Production by the Yeast Komagataella pastoris. Applied Sciences. 2021; 11(12):5516. https://doi.org/10.3390/app11125516
Chicago/Turabian StyleAraújo, Diana, Tatiana Costa, and Filomena Freitas. 2021. "Biovalorization of Lignocellulosic Materials for Xylitol Production by the Yeast Komagataella pastoris" Applied Sciences 11, no. 12: 5516. https://doi.org/10.3390/app11125516
APA StyleAraújo, D., Costa, T., & Freitas, F. (2021). Biovalorization of Lignocellulosic Materials for Xylitol Production by the Yeast Komagataella pastoris. Applied Sciences, 11(12), 5516. https://doi.org/10.3390/app11125516