The Role of Prediabetes as a Predictive Factor for the Outcomes in Patients with STEMI. Which Is the Right Range of Glycated Hemoglobin to Adopt in This Setting?
Abstract
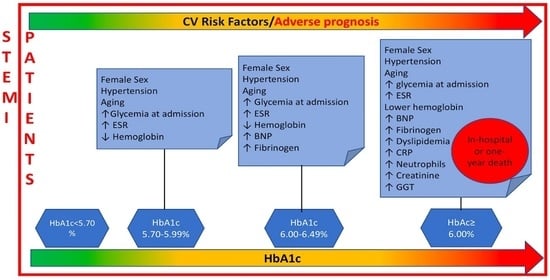
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Characteristics and Data Acquisition
2.2. Measurements and Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Correlates of HbA1c as Qualitative Variable
3.3. Correlates of HbA1c as Continuous Variable
3.4. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| preT2D | Prediabetes |
| T2D | Type 2 diabetes |
| CV | Cardiovascular |
| STEMI | Acute ST-elevation Myocardial Infarction |
| HbA1c | Glycated Hemoglobin |
| ADA | American Diabetes Association |
| WHO | World Health Organization |
| FPB | Fasting Blood Glucose |
| OGTT | Oral glucose Test Tolerance |
| EF | Ejection Fraction |
| LV | Left Ventricle |
| LVEF | Left Ventricular ejection Fraction |
| CRP | C Reactive Protein |
| ESR | Erythrocyte Sedimentation Rate |
| BNP | Brain Natriuretic Peptide |
| GGT | Gamma Glutamyl Transferase |
| BMI | Body Mass Index |
| AMI | Acute myocardial infarction |
References
- Otten, R.; Kline-Rogers, E. Impact of pre-diabetic state on clinical outcomes in patients with acute coronary syndrome. Heart 2005, 91, 1466–1468. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeli, F.; Reboldi, G. Hyperglycemia in acute coronary syndromes: From mechanisms to prognostic implications. Ther. Adv. Cardiovasc. Dis. 2015, 9, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Abdul-Ghani, M. Review of methods for detecting glycemic disorders. Diabetes Res. Clin. Pract. 2020, 165, 108233. [Google Scholar] [CrossRef]
- Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.; et al. Recommendations for chamber quantification. Eur. J. Echocardiogr. 2006, 7, 79–108. [Google Scholar] [CrossRef]
- World Health Organization; IDF. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Soler, N.G.; Frank, S. Value of glycosylated hemoglobin measurements after acute myocardial infarction. JAMA 1981, 246, 1690–1693. [Google Scholar] [CrossRef]
- Selvin, E.; Steffes, M.W.; Zhu, H.; Matsushita, K.; Wagenknecht, L.; Pankow, J.; Coresh, J.; Brancati, F.L. Glycated Hemoglobin, Diabetes, and Cardiovascular Risk in Nondiabetic Adults. N. Engl. J. Med. 2010, 362, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Giraldez, R.R.; Clare, R.M. Prevalence and clinical outcomes of undiagnosed diabetes mellitus and prediabetes among patients with high-risk non-ST-segment elevation acute coronary syndrome. Am. Heart J. 2013, 165, 918–925. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes Prevention Program. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Tabák, A.G.; Herder, C. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [Green Version]
- Cheung, B.M.; Li, C. Diabetes and hypertension: Is there a common metabolic pathway? Curr. Atheroscler. Rep. 2012, 14, 160–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFronzo, R.A.; Ferrannini, E. Insulin resistance: A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef]
- Shahim, B.; Gyberg, V. Undetected dysglycaemia common in primary care patients treated for hypertension and/or dyslipidaemia: On the need for a screening strategy in clinical practice. A report from EUROASPIRE IV a registry from the EuroObservational Research Programme of the European Society of Cardiology. Cardiovasc. Diabetol. 2018, 17, 21. [Google Scholar] [PubMed] [Green Version]
- Farhan, S.; Redfors, B. Impact of Pre-Diabetes on Coronary Plaque Composition and Clinical Outcome in Patients with Acute Coronary Syndromes: An Analysis from the PROSPECT Study. JACC Cardiovasc. Imaging 2019, 12, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Lehto, S. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef]
- Lenzen, M.; Ryden, L.; Öhrvik, J.; Bartnik, M.; Malmberg, K.; Reimer, W.S.O.; Simoons, M.L.; on Behalf of the Euro Heart Survey Investigators. Diabetes known or newly detected, but not impaired glucose regulation, has a negative influence on 1-year outcome in patients with coronary artery disease: A report from the Euro Heart Survey on diabetes and the heart. Eur. Heart J. 2006, 27, 2969–2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuusisto, J.; Mykkanen, L. NIDDM and its metabolic control predict coronary heart disease in elderly subjects. Diabetes 1994, 43, 960–967. [Google Scholar] [CrossRef]
- Lindsey, J.B.; House, J.A.; Kennedy, K.F.; Marso, S.P. Diabetes Duration Is Associated with Increased Thin-Cap Fibroatheroma Detected by Intravascular Ultrasound With Virtual Histology. Circ. Cardiovasc. Interv. 2009, 2, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, S.; Liao, Y.; Sinha, D.; Cao, G.; McGee, D.L.; Lipsitz, S.R. Sex Differences in the Effect of Diabetes Duration on Coronary Heart Disease Mortality. Arch. Intern. Med. 2005, 165, 430–435. [Google Scholar] [CrossRef] [Green Version]
- Stolar, M. Glycemic control and complications in type 2 diabetes mellitus. Am. J. Med. 2010, 123, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Dankner, R.; Danoff, A. Can ‘personalized diagnostics’ promote earlier intervention for dysglycaemia? Hypothesis ready for testing. Diabetes Metab. Res. Rev. 2010, 26, 7–9. [Google Scholar] [CrossRef] [PubMed]



| HbA1c <5.7% | HbA1c 5.7–5.99% | HbA1c 6–6.49% | HbA1c >6.49% | ||
|---|---|---|---|---|---|
| Total population | 875 (52) | 290 (17) | 196 (12) | 320 (19) | |
| Females | 183 (21) | 99 (34) | 71 (36) | 111 (35) | |
| Males | 692 (79) | 191 (66) | 120 (64) | 209 (65) | |
| Age | |||||
| <66 years (50th percentile) | 485 (55) | 120 (41) | 68 (35) | 117 (37) | |
| 66–77 years (75th percentile) | 221 (25) | 76 (26) | 57 (29) | 97 (30) | |
| >77 years | 167 (20) | 94 (32) | 71 (36) | 106 (33) | |
| CV risk factors | |||||
| Hypertension | 431 (50) | 177 (61) | 129 (66) | 220 (69) | |
| Dyslipidemia | 320 (37) | 121 (42) | 79 (40) | 156 (49) | |
| Current/ex smoking habit | 419 (48) | 111 (38) | 78 (40) | 108 (34) | |
| Ejection fraction (%) | 46 ± 9 | 44 ± 9 | 44 ± 9 | 44 ± 9 | |
| Body mass index (kg/m2) | 26 ± 4 | 27 ± 4 | 27 ± 5 | 29 ± 5 | |
| Laboratory parameters | |||||
| Creatinine (mg/dL) | 1 ± 0.6 | 1.1 ± 0.6 | 1.2 ± 0.7 | 1.4 ± 1.1 | |
| Glycemia (mg/dL) | 111 ± 29 | 125 ± 37 | 141 ± 48 | 195 ± 85 | |
| Brain natriuretic peptide (pg/mL) | 211 ± 353 | 270 ± 447 | 353 ± 665 | 350 ± 514 | |
| Fibrinogen (mg/dL) | 327 ± 110 | 333 ± 110 | 354 ± 111 | 358 ± 115 | |
| Hemoglobin (g/dL) | 14 ± 2 | 13 ± 2 | 13 ± 2 | 13 ± 2 | |
| C reactive protein (mg/dL) | 2 ± 4 | 2.1 ± 4 | 2.5 ± 5 | 2.7 ± 4 | |
| Gamma glutamyltransferase (UI/L) | 31 ± 36 | 31 ± 27 | 33 ± 36 | 36 ± 32 | |
| Monocytes (109/L) | 0.7 ± 0.4 | 0.7 ± 0.4 | 0.7 ± 0.5 | 0.7 ± 0.4 | |
| Neutrophils (109/L) | 8.6 ± 3.6 | 8.8 ± 3.7 | 9.2 ± 3.7 | 9.6 ± 4.2 | |
| Erythrocyte sedimentation rate (mm/h) | 19 ± 18 | 25 ± 21 | 25 ± 20 | 27 ± 24 |
| HbA1c% | p | ||
|---|---|---|---|
| Total population | 5.9 ± 1.1 | ||
| Females | 6.1 ± 1.2 | ||
| Males | 5.9 ± 1.1 | <0.001 | |
| Age | |||
| <66 years (50th percentile) | 5.9 ± 1.1 | ||
| 66–77 years (75th percentile) | 6 ± 1.1 | ||
| >77 years | 6.1 ± 0.9 | <0.001 | |
| CV risk factors | |||
| No-hypertension | 5.8 ± 1 | ||
| Hypertension | 6.1 ± 1.2 | <0.001 | |
| No-dyslipidemia | 5.9 ± 1.1 | ||
| Dyslipidemia | 6.1 ± 1.1 | ≤0.01 | |
| No-smoking habit | 6 ± 1.1 | ||
| Current/ex smoking habit | 5.8 ± 1 | <0.001 | |
| Ejection fraction (%) | r = −0.1 | ≤0.01 | |
| Body mass index (kg/m2) | r = 0.2 | <0.001 | |
| Laboratory parameters | |||
| Creatinine (mg/dL) | r = 0.1 | <0.001 | |
| Glycemia (mg/dL) | r = 0.6 | <0.001 | |
| Brain natriuretic peptide (pg/mL) | r = 0.1 | <0.001 | |
| Fibrinogen (mg/dL) | r = 0.1 | <0.001 | |
| Hemoglobin (g/dL) | r = −0.1 | <0.01 | |
| C reactive protein (mg/dL) | r = 0.2 | <0.001 | |
| Gamma glutamyltransferase (UI/L) | r = 0.1 | <0.001 | |
| Monocytes (109/L) | r = 0.02 | ns | |
| Neutrophils (109/L) | r = 0.1 | <0.001 | |
| Erythrocyte sedimentation rate (mm/h) | r = 0.1 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzianagnostou, K.; Guiducci, L.; Paradossi, U.; De Caterina, A.R.; Mazzone, A.; Berti, S.; Vassalle, C. The Role of Prediabetes as a Predictive Factor for the Outcomes in Patients with STEMI. Which Is the Right Range of Glycated Hemoglobin to Adopt in This Setting? Appl. Sci. 2021, 11, 5518. https://doi.org/10.3390/app11125518
Chatzianagnostou K, Guiducci L, Paradossi U, De Caterina AR, Mazzone A, Berti S, Vassalle C. The Role of Prediabetes as a Predictive Factor for the Outcomes in Patients with STEMI. Which Is the Right Range of Glycated Hemoglobin to Adopt in This Setting? Applied Sciences. 2021; 11(12):5518. https://doi.org/10.3390/app11125518
Chicago/Turabian StyleChatzianagnostou, Kyriazoula, Letizia Guiducci, Umberto Paradossi, Alberto Ranieri De Caterina, Annamaria Mazzone, Sergio Berti, and Cristina Vassalle. 2021. "The Role of Prediabetes as a Predictive Factor for the Outcomes in Patients with STEMI. Which Is the Right Range of Glycated Hemoglobin to Adopt in This Setting?" Applied Sciences 11, no. 12: 5518. https://doi.org/10.3390/app11125518
APA StyleChatzianagnostou, K., Guiducci, L., Paradossi, U., De Caterina, A. R., Mazzone, A., Berti, S., & Vassalle, C. (2021). The Role of Prediabetes as a Predictive Factor for the Outcomes in Patients with STEMI. Which Is the Right Range of Glycated Hemoglobin to Adopt in This Setting? Applied Sciences, 11(12), 5518. https://doi.org/10.3390/app11125518