Preliminary Design of a Self-Sufficient Electrical Storage System Based on Electrolytic Hydrogen for Power Supply in a Residential Application
Abstract
:1. Introduction
2. Methodology
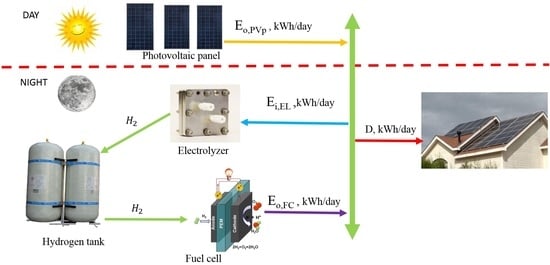
2.1. General Design Considerations
2.2. Design Path
3. Results and Discussions
3.1. Photovoltaic Panel
3.2. AEMWE Electrolyzer
3.3. Fuel Cell
3.4. Hydrogen Tank
4. Economic Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| BA | Battery |
| EEL | Consumed energy by the electrolyzer per kg of hydrogen, 45.29 kWh/kgH2 |
| I | Current, A |
| ηBA | Efficiency of battery, 0.9 |
| ηFC | Efficiency of fuel cell, 0.65 |
| D | Electrical demand, Wh/day or kWh/day |
| SEL | Electrolyzer area |
| EL | Electrolysis cell |
| E | Energy, kWh |
| EBae | Energy of the extra battery, kWh/day |
| Ee | Excess energy, kWh/day |
| BAe | Extra battery |
| FC | Fuel cell |
| HT | Hydrogen tank |
| Ei | Input energy, kWh/day |
| Ei,EL | Input energy of electrolyzer, kWh/day |
| NEL | Number of EL cells in series |
| NPV | Number of PV modules in series |
| PV1m | One photovoltaic module |
| So,EL | Optimal area of electrolyzer |
| NPV,OP | Optimal number of PV modules in series |
| Eo | Output energy, kWh/day |
| Eo,BA | Output energy of battery, kWh |
| Eo,FC | Output energy of fuel cell, kWh/day |
| Eo,PVnm | Output energy of n photovoltaic module, kWh/day; n: number of photovoltaic modules. |
| Po,FC | Output power of fuel cell, kW |
| OT | Oxygen tank |
| SPVp | Photovoltaic array area |
| SPVm | Photovoltaic module area |
| P | Power, kW |
| IMPP | PV Current at the maximum power point (MPP), A |
| VMPP | PV Voltage at the maximum power point (MPP), V |
| fEL | Sizing factor of electrolyzer |
| fPV | Sizing factor of photovoltaic panel |
| PVP | Solar PV array |
| G | Solar radiation, W/m2 |
| SSEL | Stack electrolyzer area |
| T | Time, hour or day |
| V | Voltage, V |
References
- Amores, E.; Rodríguez, J.; Carreras, C. Influence of operation parameters in the modeling of alkaline water electrolyzers for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 13063–13078. [Google Scholar] [CrossRef]
- Siegel, A.; Schott, T. Optimization of photovoltaic hydrogen production. Int. J. Hydrogen Energy 1988, 13, 659–675. [Google Scholar] [CrossRef]
- López-Fernández, E.; Gil-Rostra, J.; Espinós, J.P.; González-Elipe, A.R.; Yubero, F.; de Lucas-Consuegra, A. CuxCo3-xO4 ultra-thin film as efficient anodic catalysts for anion exchange membrane water electrolysers. J. Power Sources 2019, 415, 136–144. [Google Scholar] [CrossRef]
- López-Fernández, E.; Gil-Rostra, J.; Espinós, J.P.; González-Elipe, A.R.; De Lucas Consuegra, A.; Yubero, F. Chemistry and Electrocatalytic Activity of Nanostructured Nickel Electrodes for Water Electrolysis. ACS Catal. 2020, 10, 6159–6170. [Google Scholar] [CrossRef]
- Caravaca, A.; Sapountzi, F.M.; De Lucas-Consuegra, A.; Molina-Mora, C.; Dorado, F.; Valverde, J.L. Electrochemical reforming of ethanol-water solutions for pure H2 production in a PEM electrolysis cell. Int. J. Hydrogen Energy 2012, 37, 9504–9513. [Google Scholar] [CrossRef]
- González-Cobos, J.; Valverde, J.L.; de Lucas-Consuegra, A. Electrochemical vs. chemical promotion in the H2 production catalytic reactions. Int. J. Hydrogen Energy 2017, 42, 13712–13723. [Google Scholar] [CrossRef]
- Kelly, N.A. Hydrogen production by water electrolysis. In Advances in Hydrogen Production, Storage and Distribution; Woodhead Publishing: Cambridge, UK, 2014; ISBN 9780857097736. [Google Scholar]
- Maeda, T.; Ito, H.; Hasegawa, Y.; Zhou, Z.; Ishida, M. Study on control method of the stand-alone direct-coupling photovoltaic—Water electrolyzer. Int. J. Hydrogen Energy 2012, 37, 4819–4828. [Google Scholar] [CrossRef]
- Khalilnejad, A.; Riahy, G.H. A hybrid wind-PV system performance investigation for the purpose of maximum hydrogen production and storage using advanced alkaline electrolyzer. Energy Convers. Manag. 2014, 80, 398–406. [Google Scholar] [CrossRef]
- Chaparro, A.M.; Soler, J.; Escudero, M.J.; De Ceballos, E.M.L.; Wittstadt, U.; Daza, L. Data results and operational experience with a solar hydrogen system. J. Power Sources 2005, 144, 165–169. [Google Scholar] [CrossRef]
- Erdinc, O.; Uzunoglu, M. A new perspective in optimum sizing of hybrid renewable energy systems: Consideration of component performance degradation issue. Int. J. Hydrogen Energy 2012, 37, 10479–10488. [Google Scholar] [CrossRef]
- Sánchez, M.; Amores, E.; Rodríguez, L.; Clemente-Jul, C. Semi-empirical model and experimental validation for the performance evaluation of a 15 kW alkaline water electrolyzer. Int. J. Hydrogen Energy 2018, 43, 20332–20345. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P. Liquid Organic Hydrogen Carriers as an efficient vector for the transport and storage of renewable energy. Int. J. Hydrogen Energy 2012, 37, 18118–18132. [Google Scholar] [CrossRef]
- Reuß, M.; Grube, T.; Robinius, M.; Preuster, P.; Wasserscheid, P.; Stolten, D. Seasonal storage and alternative carriers: A flexible hydrogen supply chain model. Appl. Energy 2017, 200, 290–302. [Google Scholar] [CrossRef]
- He, T.; Pei, Q.; Chen, P. Liquid organic hydrogen carriers. J. Energy Chem. 2015, 24, 587–594. [Google Scholar] [CrossRef] [Green Version]
- García-Valverde, R.; Espinosa, N.; Urbina, A. Optimized method for photovoltaic-water electrolyser direct coupling. Int. J. Hydrogen Energy 2011, 36, 10574–10586. [Google Scholar] [CrossRef]
- Ozden, E.; Tari, I. Energy-exergy and economic analyses of a hybrid solar-hydrogen renewable energy system in Ankara, Turkey. Appl. Therm. Eng. 2016, 99, 169–178. [Google Scholar] [CrossRef]
- Gutiérrez-Martín, F.; Calcerrada, A.B.; de Lucas-Consuegra, A.; Dorado, F. Hydrogen storage for off-grid power supply based on solar PV and electrochemical reforming of ethanol-water solutions. Renew. Energy 2020, 147, 639–649. [Google Scholar] [CrossRef]
- Budak, Y.; Devrim, Y. Comparative study of PV/PEM fuel cell hybrid energy system based on methanol and water electrolysis. Energy Convers. Manag. 2019, 179, 46–57. [Google Scholar] [CrossRef]
- PV System Photovoltaic Software. Available online: https://www.pvsyst.com/ (accessed on 10 November 2020).
- Mermoud, A.; Lejeune, T. Performance Assessment of a Simulation Model for Pv Modules of Any Available Technology. In Proceedings of the 25th European Photovoltaic Solar Energy Conference, Valencia, Spain, 6–10 September 2010; pp. 6–10. [Google Scholar]
- Mermoud, A.; Villoz, A.; Wittmer, B.; Apaydin, H. ECONOMIC OPTIMIZATION OF PV SYSTEMS WITH STORAGE. 2020. PVsyst SA Route de la Maison-Carrée 30, CH 1242 Satigny, Switzerland. Available online: https://www.pvsyst.com/ (accessed on 13 October 2021).
- Zablocki, A. Energy storage: Fact sheet (2019). Eesi 2019, 2040, 1–8. [Google Scholar]
- Gibson, T.L.; Kelly, N.A. Optimization of solar powered hydrogen production using photovoltaic electrolysis devices. Int. J. Hydrogen Energy 2008, 33, 5931–5940. [Google Scholar] [CrossRef]
- Behzadi, M.S.; Niasati, M. Comparative performance analysis of a hybrid PV/FC/battery stand-alone system using different power management strategies and sizing approaches. Int. J. Hydrogen Energy 2015, 40, 538–548. [Google Scholar] [CrossRef]
- Clarke, R.E.; Giddey, S.; Ciacchi, F.T.; Badwal, S.P.S.; Paul, B.; Andrews, J. Direct coupling of an electrolyser to a solar PV system for generating hydrogen. Int. J. Hydrogen Energy 2009, 34, 2531–2542. [Google Scholar] [CrossRef]
- Al-Khori, K.; Bicer, Y.; Koç, M. Comparative techno-economic assessment of integrated PV-SOFC and PV-Battery hybrid system for natural gas processing plants. Energy 2021, 222, 119923. [Google Scholar] [CrossRef]
- Amores, E.; Sánchez, M.; Rojas, N.; Sánchez-Molina, M. Renewable hydrogen production by water electrolysis. In Sustainable Fuel Technologies Handbook; Elsevier: Amsterdam, The Netherlands, 2021; pp. 271–313. [Google Scholar]
- Zhang, X.; Ni, M.; Wang, J.; Yang, L.; Mao, X.; Su, S.; Yang, Z.; Chen, J. Configuration design and parametric optimum selection of a self-supporting PEMFC. Energy Convers. Manag. 2020, 225, 113391. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021. [Google Scholar] [CrossRef]
- Wishart, J.; Dong, Z.; Secanell, M. Optimization of a PEM fuel cell system based on empirical data and a generalized electrochemical semi-empirical model. J. Power Sources 2006, 161, 1041–1055. [Google Scholar] [CrossRef]
- Cheng, S.; Xu, L.; Wu, K.; Fang, C.; Hu, J.; Li, J.; Ouyang, M. Optimal warm-up control strategy of the PEMFC system on a city bus aimed at improving efficiency. Int. J. Hydrogen Energy 2017, 42, 11632–11643. [Google Scholar] [CrossRef]
- Authayanun, S.; Wiyaratn, W.; Assabumrungrat, S.; Arpornwichanop, A. Theoretical analysis of a glycerol reforming and high-temperature PEMFC integrated system: Hydrogen production and system efficiency. Fuel 2013, 105, 345–352. [Google Scholar] [CrossRef]
- Witkowski, A.; Rusin, A.; Majkut, M.; Stolecka, K. Comprehensive analysis of hydrogen compression and pipeline transportation from thermodynamics and safety aspects. Energy 2017, 141, 2508–2518. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H. wen Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Castañeda, M.; Cano, A.; Jurado, F.; Sánchez, H.; Fernández, L.M. Sizing optimization, dynamic modeling and energy management strategies of a stand-alone PV/hydrogen/battery-based hybrid system. Int. J. Hydrogen Energy 2013, 38, 3830–3845. [Google Scholar] [CrossRef]
- de Andrade, G.A.; Mendes, P.R.C.; García-Clúa, J.G.; Normey-Rico, J.E. Control of a grid assisted PV-H2 production system: A comparative study between optimal control and hybrid MPC. J. Process Control 2020, 92, 220–233. [Google Scholar] [CrossRef]
- Amores, E.; Rodríguez, J.; Oviedo, J.; De Lucas-Consuegra, A. Development of an operation strategy for hydrogen production using solar PV energy based on fluid dynamic aspects. Open Eng. 2017, 7, 141–152. [Google Scholar] [CrossRef]
- Pellow, M.A.; Emmott, C.J.M.; Barnhart, C.J.; Benson, S.M. Hydrogen or batteries for grid storage? A net energy analysis. Energy Environ. Sci. 2015, 8, 1938–1952. [Google Scholar] [CrossRef] [Green Version]
- Motealleh, B.; Liu, Z.; Masel, R.I.; Sculley, J.P.; Richard Ni, Z.; Meroueh, L. Next-generation anion exchange membrane water electrolyzers operating for commercially relevant lifetimes. Int. J. Hydrogen Energy 2021, 46, 3379–3386. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Liu, H.; Liu, Q.; Tang, J.; Li, Z. Battery degradation model and multiple-indicators based lifetime estimator for energy storage system design and operation: Experimental analyses of cycling-induced aging. Electrochim. Acta 2021, 384, 138294. [Google Scholar] [CrossRef]
- Harrison, K. 2nd International Workshop Durability and Degradation Issues in PEM Electrolysis Cells and its Components Life-Time Prediction of PEM Water Electrolysis Stacks Coupled with RES Transportation. Johns. Matthey Technol. Rev. 2016, 47–51. [Google Scholar] [CrossRef]
- Hollmuller, P.; Joubert, J.M.; Lachal, B.; Yvon, K. Evaluation of a 5 kWp photovoltaic hydrogen production and storage installation for a residential home in Switzerland. Int. J. Hydrogen Energy 2000, 25, 97–109. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.-J.; Yuan, S.-H.; Wuu, D.-S.; Wu, W.-Y.; Zhang, S. Improvement in the Figure of Merit of ITO-Metal-ITO Sandwiched Films on Poly Substrate by High-Power Impulse Magnetron Sputtering. Coatings 2021, 11, 144. [Google Scholar] [CrossRef]
- Ababneh, A.; Dagamseh, A.M.K.; Albataineh, Z.; Tantawi, M.; Al-Bataineh, Q.M.; Telfah, M.; Zengerle, T.; Seidel, H. Optical and structural properties of aluminium nitride thin-films synthesized by DC-magnetron sputtering technique at different sputtering pressures. Microsyst. Technol. 2021, 4. [Google Scholar] [CrossRef]
- Cho, S. Properties of Eu-doped—YVO 4 thin films grown on glass substrates by radio - frequency magnetron sputtering. Appl. Phys. A 2021, 127, 161. [Google Scholar] [CrossRef]
- Liu, Z.; Sajjad, S.D.; Gao, Y.; Yang, H.; Kaczur, J.J.; Masel, R.I. The effect of membrane on an alkaline water electrolyzer. Int. J. Hydrogen Energy 2017, 42, 29661–29665. [Google Scholar] [CrossRef]
- Di Profio, P.; Arca, S.; Rossi, F.; Filipponi, M. Comparison of hydrogen hydrates with existing hydrogen storage technologies: Energetic and economic evaluations. Int. J. Hydrogen Energy 2009, 34, 9173–9180. [Google Scholar] [CrossRef]
- Ozaki, M.; Tomura, S.; Ohmura, R.; Mori, Y.H. Comparative study of large-scale hydrogen storage technologies: Is hydrate-based storage at advantage over existing technologies? Int. J. Hydrogen Energy 2014, 39, 3327–3341. [Google Scholar] [CrossRef]
- Niermann, M.; Timmerberg, S.; Drünert, S.; Kaltschmitt, M. Liquid Organic Hydrogen Carriers and alternatives for international transport of renewable hydrogen. Renew. Sustain. Energy Rev. 2021, 135, 110171. [Google Scholar] [CrossRef]
- Mench, M.M. Fuel Cell Engines; Chapter 8; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Enapter Website. Available online: https://handbook.enapter.com/electrolyser/el21/downloads/Enapter_Datasheet_EL21_EN.pdf (accessed on 10 December 2020).
- Code ASME. BPVC, Section VIII, DIV 1. 2015. Available online: https://www.asme.org/wwwasmeorg/media/resourcefiles/shop/standards/newreleases/asme-bpvc-brochure.pdf (accessed on 20 December 2020).
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Mijalev, M.F. Cálculo y Diseño de Máquinas y Aparatos de la Industria Química; Mijalev, M.F., Tretiakov, N.P., Milchenko, A.l., Zobnin, Y.V., Eds.; Yneshtorgizdat: Moscú, Russia, 1986. [Google Scholar]
- Moss, D.R. Pressure Vessel Design Manual: Illustrated Procedures for Solving Every Mayor Pressure Vessel Design Problem, 2nd ed.; Gulf Publishing Company: Houston, TX, USA, 1987. [Google Scholar]
- Megyesy, E.F. Manual de Recipientes a Presión: Diseño y Cálculo; Limusa: Mexico City, Mexico, 1992. [Google Scholar]
- Sinnott, R.K. Chemical Engineering Design; Elsevier Butterworth-Heinemann: Oxford, UK, 2005. [Google Scholar]
- Nižetić, S.; Tolj, I.; Papadopoulos, A.M. Hybrid energy fuel cell based system for household applications in a Mediterranean climate. Energy Convers. Manag. 2015, 105, 1037–1045. [Google Scholar] [CrossRef]
- Nižetić, S.; Duić, N.; Papadopulos, A.M.; Tina, G.M.; Grubišić-Čabo, F. Energy efficiency evaluation of a hybrid energy system for building applications in a Mediterranean climate and its feasibility aspect. Energy 2015, 90, 1171–1179. [Google Scholar] [CrossRef]
- Domingos, R.M.A.; Pereira, F.O.R. Comparative cost-benefit analysis of the energy efficiency measures and photovoltaic generation in houses of social interest in Brazil. Energy Build. 2021, 243, 111013. [Google Scholar] [CrossRef]
- Isa, N.M.; Das, H.S.; Tan, C.W.; Yatim, A.H.M.; Lau, K.Y. A techno-economic assessment of a combined heat and power photovoltaic/fuel cell/battery energy system in Malaysia hospital. Energy 2016, 112, 75–90. [Google Scholar] [CrossRef]
- Budak, Y.; Devrim, Y. Investigation of micro-combined heat and power application of PEM fuel cell systems. Energy Convers. Manag. 2018, 160, 486–494. [Google Scholar] [CrossRef]
- Uhm, S.; Jeon, H.; Kim, T.J.; Lee, J. Clean hydrogen production from methanol-water solutions via power-saved electrolytic reforming process. J. Power Sources 2012, 198, 218–222. [Google Scholar] [CrossRef]
- Gökçek, M.; Kale, C. Optimal design of a Hydrogen Refuelling Station (HRFS) powered by Hybrid Power System. Energy Convers. Manag. 2018, 161, 215–224. [Google Scholar] [CrossRef]
- Marocco, P.; Ferrero, D.; Lanzini, A.; Santarelli, M. Optimal design of stand-alone solutions based on RES + hydrogen storage feeding off-grid communities. Energy Convers. Manag. 2021, 238, 114147. [Google Scholar] [CrossRef]
- Basu, S.; John, A.; Akshay; Kumar, A. Design and feasibility analysis of hydrogen based hybrid energy system: A case study. Int. J. Hydrogen Energy 2021, 46, 34574–34586. [Google Scholar] [CrossRef]







| t | Eo,PV30m | Ee,PV30m | Eo,BA | Ei,EL | Ei,BAe | Ee,PVn |
|---|---|---|---|---|---|---|
| (Days) | (kWh/Day) | (kWh/Day) | (kWh/Day) | (kWh/Day) | (kWh/Day) | (kWh/Day) |
| 1 | 14 | 6.365 | 5.729 | 17.314 | 1.731 | 19.045 |
| 2 | 6.81 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 3 | 4.613 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 4 | 7.021 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 5 | 13.65 | 6.015 | 5.414 | 16.362 | 1.636 | 17.998 |
| 6 | 6.598 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 7 | 1.697 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 8 | 5.79 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 9 | 7.528 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 10 | 4.938 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 11 | 4.883 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 12 | 15.78 | 8.145 | 7.331 | 22.155 | 2.216 | 24.371 |
| 13 | 16.51 | 8.875 | 7.988 | 24.141 | 2.414 | 26.555 |
| 14 | 4.798 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 15 | 10.5 | 2.865 | 2.579 | 7.793 | 0.779 | 8.572 |
| 16 | 10.14 | 2.505 | 2.255 | 6.814 | 0.681 | 7.495 |
| 17 | 18.53 | 10.895 | 9.806 | 29.636 | 2.964 | 32.599 |
| 18 | 6.235 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 19 | 8.691 | 1.056 | 0.950 | 2.872 | 0.287 | 3.160 |
| 20 | 3.597 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 21 | 11.45 | 3.815 | 3.434 | 10.377 | 1.038 | 11.415 |
| 22 | 10.07 | 2.435 | 2.192 | 6.623 | 0.662 | 7.286 |
| 23 | 5.58 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 24 | 11.45 | 3.815 | 3.434 | 10.377 | 1.038 | 11.415 |
| 25 | 7.557 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 26 | 8.529 | 0.894 | 0.805 | 2.432 | 0.243 | 2.675 |
| 27 | 1.435 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 28 | 1.357 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 29 | 1.593 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 30 | 17.85 | 10.215 | 9.194 | 27.786 | 2.779 | 30.565 |
| 31 | 20.24 | 12.605 | 11.345 | 34.287 | 3.429 | 37.716 |
| System | Component | Data Specification |
|---|---|---|
| EL-FC | Photovoltaic Panel | |
| Size (Wp/module) | 109 | |
| Lifetime (years) | 20 | |
| Capital Cost (€) | 3052 | |
| O&M Cost (€/year) | 5 | |
| Electrolyzer | ||
| Size (cm2) | 50 | |
| Lifetime (years) | 20 | |
| Capital Cost (€) | 5493 | |
| O&M Cost (€/year) | 10 | |
| Fuel Cell | ||
| Size (W) | 1013 | |
| Lifetime | 15,000 | |
| Capital Cost (€) | 2436 | |
| O&M Cost (€/year) | 4 | |
| Hydrogen Tank | ||
| Size (m3) | 0.91 | |
| Lifetime (years) | 20 | |
| Capital Cost (€) | 1846 | |
| O&M Cost (€/year) | 3 | |
| Conventional | Photovoltaic Panel | |
| Size (Wp/module) | 106 | |
| Lifetime (years) | 20 | |
| Capital Cost (€) | 3562 | |
| O&M Cost (€/year) | 6 | |
| Battery | ||
| Nominal Capacity (kWh) | 32.3 | |
| Lifetime (years) | 1 | |
| Capital Cost (€) | 7273 | |
| O&M Cost (€/year) | 12 |
| System | Component | Cost (€) | Total (€) | NPV (€) | IRR (%) | LCOE (€/kWh) |
|---|---|---|---|---|---|---|
| EL-FC | Photovoltaic Panel | 3052 | 12,827 | 660 | 10.61 | 0.541 |
| Electrolyzer | 5493 | |||||
| Fuel Cell | 2436 | |||||
| Hydrogen Tank | 1846 | |||||
| BATTERY | Photovoltaic Panel | 3562 | 10,835 | 2164 | 12.31 | 0.534 |
| Battery | 7273 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Sacedón, C.; López-Fernández, E.; de la Osa-Puebla, A.R.; Dorado-Fernández, F.; Amores-Vera, E.; de Lucas-Consuegra, A. Preliminary Design of a Self-Sufficient Electrical Storage System Based on Electrolytic Hydrogen for Power Supply in a Residential Application. Appl. Sci. 2021, 11, 9582. https://doi.org/10.3390/app11209582
Gómez-Sacedón C, López-Fernández E, de la Osa-Puebla AR, Dorado-Fernández F, Amores-Vera E, de Lucas-Consuegra A. Preliminary Design of a Self-Sufficient Electrical Storage System Based on Electrolytic Hydrogen for Power Supply in a Residential Application. Applied Sciences. 2021; 11(20):9582. https://doi.org/10.3390/app11209582
Chicago/Turabian StyleGómez-Sacedón, Celia, Ester López-Fernández, Ana Raquel de la Osa-Puebla, Fernando Dorado-Fernández, Ernesto Amores-Vera, and Antonio de Lucas-Consuegra. 2021. "Preliminary Design of a Self-Sufficient Electrical Storage System Based on Electrolytic Hydrogen for Power Supply in a Residential Application" Applied Sciences 11, no. 20: 9582. https://doi.org/10.3390/app11209582
APA StyleGómez-Sacedón, C., López-Fernández, E., de la Osa-Puebla, A. R., Dorado-Fernández, F., Amores-Vera, E., & de Lucas-Consuegra, A. (2021). Preliminary Design of a Self-Sufficient Electrical Storage System Based on Electrolytic Hydrogen for Power Supply in a Residential Application. Applied Sciences, 11(20), 9582. https://doi.org/10.3390/app11209582