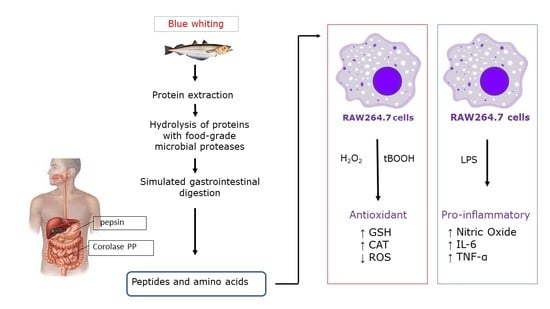
Blue Whiting Protein Hydrolysates Exhibit Antioxidant and Immunomodulatory Activities in Stimulated Murine RAW264.7 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Cell Culture and Sample Preparation
2.4. DPPH Activity
2.5. ORAC Activity
2.6. FRAP Activity
2.7. Cell Viability
2.8. ROS Production
2.9. GSH Content
2.10. CAT Activity
2.11. NO Secretion
2.12. Cytokine Secretion
2.13. Statistical Analysis
3. Results and Discussion
3.1. Noncellular In Vitro Antioxidant Activity
3.2. Effect of BWSPHs on RAW264.7 Cell Viability
3.3. Cellular Antioxidant Activity
3.4. Cellular Immunomodulatory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, C.; Chen, Y.; Zheng, B. Purification and Characterization of Antioxidant Peptides of Pseudosciaena crocea Protein Hydrolysates. Molecules 2016, 22, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.-M.; Wang, Y.-M.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Antioxidant Peptides from the Protein Hydrolysate of Monkfish (Lophius litulon) Muscle: Purification, Identification, and Cytoprotective Function on HepG2 Cells Damage by H2O2. Mar. Drugs 2020, 18, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, J.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Bioactive Peptides from Cartilage Protein Hydrolysate of Spotless Smoothhound and Their Antioxidant Activity In Vitro. Mar. Drugs 2018, 16, 100. [Google Scholar] [CrossRef] [Green Version]
- Fernando, I.P.S.; Park, S.Y.; Han, E.J.; Kim, H.-S.; Kang, D.-S.; Je, J.-Y.; Ahn, C.-B.; Ahn, G. Isolation of an antioxidant peptide from krill protein hydrolysates as a novel agent with potential hepatoprotective effects. J. Funct. Foods 2020, 67, 103889. [Google Scholar] [CrossRef]
- Bkhairia, I.; Dhibi, S.; Nasri, R.; Elfeki, A.; Hfaiyedh, N.; Ben Amara, I.; Nasri, M. Bioactive properties: Enhancement of hepatoprotective, antioxidant and DNA damage protective effects of golden grey mullet protein hydrolysates against paracetamol toxicity. RSC Adv. 2018, 8, 23230–23240. [Google Scholar] [CrossRef] [Green Version]
- Gómez, L.J.; Gómez, N.A.; Zapata, J.E.; López-García, G.; Cilla, A.; Alegría, A. In-vitro antioxidant capacity and cytoprotective/cytotoxic effects upon Caco-2 cells of red tilapia (Oreochromis spp.) viscera hydrolysates. Food Res. Int. 2019, 120, 52–61. [Google Scholar] [CrossRef]
- Heffernan, S.; Giblin, L.; O’Brien, N. Assessment of the biological activity of fish muscle protein hydrolysates using in vitro model systems. Food Chem. 2021, 359, 129852. [Google Scholar] [CrossRef] [PubMed]
- Kangsanant, S.; Thongraung, C.; Jansakul, C.; Murkovic, M.; Seechamnanturakit, V. Purification and characterisation of antioxidant and nitric oxide inhibitory peptides from Tilapia (Oreochromis niloticus) protein hydrolysate. Int. J. Food Sci. Technol. 2014, 50, 660–665. [Google Scholar] [CrossRef]
- Da Rocha, M.; Alemán, A.; Baccan, G.C.; López-Caballero, M.E.; Gómez-Guillén, C.; Montero, P.; Prentice, C. Anti-Inflammatory, Antioxidant, and Antimicrobial Effects of Underutilized Fish Protein Hydrolysate. J. Aquat. Food Prod. Technol. 2018, 27, 592–608. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.-C.; Jeon, Y.-J. Anti-inflammatory effect of enzymatic hydrolysates from Styela clavaflesh tissue in lipopolysaccharide-stimulated RAW 264.7 macrophages andin vivozebrafish model. Nutr. Res. Pr. 2015, 9, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Sung, N.-Y.; Jung, P.-M.; Yoon, M.; Kim, J.-S.; Choi, J.-I.; Jeong, H.G.; Lee, J.-W.; Kim, J.-H. Anti-inflammatory effect of sweetfish-derived protein and its enzymatic hydrolysate on LPS-induced RAW264.7 cells via inhibition of NF-κB transcription. Fish. Sci. 2012, 78, 381–390. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N. Antioxidant, immunomodulatory and antiproliferative effects of gelatin hydrolysate from unicorn leatherjacket skin. J. Sci. Food Agric. 2015, 96, 3220–3226. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Cho, Y.-S.; Je, J.-Y. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015, 168, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Toopcham, T.; Mes, J.J.; Wichers, H.J.; Yongsawatdigul, J. Immunomodulatory activity of protein hydrolysates derived from Virgibacillus halodenitrificans SK1-3-7 proteinase. Food Chem. 2017, 224, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, X.; Lin, L.; Ding, G.; Yu, F. Immunomodulatory Activity of Low Molecular-Weight Peptides from Nibea japonica in RAW264.7 Cells via NF-κB Pathway. Mar. Drugs 2019, 17, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskaran, N.; Shukla, S.; Kanwal, R.; Srivastava, J.K.; Gupta, S. Induction of heme oxygenase-1 by chamomile protects murine macrophages against oxidative stress. Life Sci. 2012, 90, 1027–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Moreno, P.J.; Gálvez, A.R.P.; Carpio, F.J.E.; Ruiz-Quesada, C.; Pérez-Morilla, A.I.; Augustin, O.M.; Guadix, A.; Guadix, E.M. Functional, bioactive and antigenicity properties of blue whiting protein hydrolysates: Effect of enzymatic treatment and degree of hydrolysis. J. Sci. Food Agric. 2016, 97, 299–308. [Google Scholar] [CrossRef]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. Characterization of protein hydrolysates from blue whiting (Micromesistius poutassou) and their application in beverage fortification. Food Chem. 2017, 245, 698–706. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.; FitzGerald, R.J. Blue whiting (Micromesistius poutassou) muscle protein hydrolysate with in vitro and in vivo antidiabetic properties. J. Funct. Foods 2018, 40, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Cudennec, B.; Ravallec-Plé, R.; Courois, E.; Fouchereau-Peron, M. Peptides from fish and crustacean by-products hydrolysates stimulate cholecystokinin release in STC-1 cells. Food Chem. 2008, 111, 970–975. [Google Scholar] [CrossRef]
- Cudennec, B.; Fouchereau-Peron, M.; Ferry, F.; Duclos, E.; Ravallec, R. In vitro and in vivo evidence for a satiating effect of fish protein hydrolysate obtained from blue whiting (Micromesistius poutassou) muscle. J. Funct. Foods 2012, 4, 271–277. [Google Scholar] [CrossRef]
- Nobile, V.; Duclous, E.; Michelotti, A.; Bizzaro, G.; Negro, M.; Soisson, F. Supplementation with a fish protein hydrolysate (Micromesistius poutassou): Effects on body weight, body composition, and CCK/GLP-1 secretion. Food Nutr. Res. 2016, 60, 29857. [Google Scholar] [CrossRef] [Green Version]
- Grainger, J.R.; Konkel, J.E.; Zangerle-Murray, T.; Shaw, T.N. Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch. 2017, 469, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Harnedy-Rothwell, P.A.; Khatib, N.; Sharkey, S.; Lafferty, R.A.; Gite, S.; Whooley, J.; O’Harte, F.P.M.; FitzGerald, R.J. Physicochemical, Nutritional and In Vitro Antidiabetic Characterisation of Blue Whiting (Micromesistius poutassou) Protein Hydrolysates. Mar. Drugs 2021, 19, 383. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harnedy, P.; Fitzgerald, R.J. In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. Environ. Boil. Fishes 2013, 25, 1793–1803. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Smith, P.; Krohn, R.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.; Goeke, N.; Olson, B.; Klenk, D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Kenny, O.M.; McCarthy, C.M.; Brunton, N.; Hossain, M.B.; Rai, D.; Collins, S.G.; Jones, P.W.; Maguire, A.; O’Brien, N.M. Anti-inflammatory properties of potato glycoalkaloids in stimulated Jurkat and Raw 264.7 mouse macrophages. Life Sci. 2013, 92, 775–782. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, S.; Rezaei, M.; Asgharzade, S.; Nikoo, M.; Rafieia-Kopai, M. Antioxidant and cytotoxic properties of protein hydrolysates obtained from enzymatic hydrolysis of Klunzinger’s mullet (Liza klunzingeri) muscle. Braz. J. Pharm. Sci. 2019, 55. [Google Scholar] [CrossRef]
- Kang, P.Y.; Ishak, N.H.; Sarbon, N.M. Optimization of enzymatic hydrolysis of shortfin scad (Decapterus macrosoma) myofibrillar protein with antioxidant effect using alcalase. Int. Food Res. J. 2018, 25, 1808–1817. [Google Scholar]
- Parvathy, U.; Zynudheen, A.A.; Panda, S.K.; Jeyakumari, A.; Anandan, R. Extraction of Protein from Yellowfin Tuna (Thunnus albacares) Waste by Enzymatic Hydrolysis and its Characterization. Fish. Technol. 2016, 53, 115–124. [Google Scholar]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited review: Whey proteins as antioxidants and promoters of cellular antioxidant pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef] [Green Version]
- Corrochano, A.R.; Sariçay, Y.; Arranz, E.; Kelly, P.M.; Buckin, V.; Giblin, L. Comparison of antioxidant activities of bovine whey proteins before and after simulated gastrointestinal digestion. J. Dairy Sci. 2019, 102, 54–67. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Luo, Y.; Shen, H.; You, J. Antioxidant activities and functional properties of grass carp (Ctenopharyngodon idellus) protein hydrolysates. J. Sci. Food Agric. 2011, 92, 292–298. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.; Fitzgerald, R.J. Angiotensin Converting Enzyme and Dipeptidyl Peptidase-IV Inhibitory, and Antioxidant Activities of a Blue Mussel (Mytilus edulis) Meat Protein Extract and Its Hydrolysates. J. Aquat. Food Prod. Technol. 2016, 25, 1221–1233. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.; FitzGerald, R.J. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chem. 2016, 218, 396–405. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, M.W.; Nelson, J.; Harrison, G.; Forman, H.J. Effects of t-butyl hydroperoxide on NADPH, glutathione, and the respiratory burst of rat alveolar macrophages. Arch. Biochem. Biophys. 1985, 243, 325–331. [Google Scholar] [CrossRef]
- Davies, M.J. Detection of peroxyl and alkoxyl radicals produced by reaction of hydroperoxides with rat liver microsomal fractions. Biochem. J. 1989, 257, 603–606. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-L.; Wang, C.-J.; Tsai, Y.-Y.; Liu, C.-L.; Hwang, J.-M.; Tseng, T.-H. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver. Arch. Toxicol. 2000, 74, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Mendis, E.; Rajapakse, A.N.; Kim, S.-K. Antioxidant Properties of a Radical-Scavenging Peptide Purified from Enzymatically Prepared Fish Skin Gelatin Hydrolysate. J. Agric. Food Chem. 2004, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-F.; Gong, F.; Zhang, Y.Y.; Li, C.-Y.; Zhou, C.-X.; Hong, P.-Z.; Sun, S.-L.; Qian, Z.-J. Preventive Effect of YGDEY from Tilapia Fish Skin Gelatin Hydrolysates against Alcohol-Induced Damage in HepG2 Cells through ROS-Mediated Signaling Pathways. Nutrients 2019, 11, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Tong, X.; Qi, B.; Wang, Z.; Li, Y.; Sui, X.; Jiang, L. Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J. Funct. Foods 2018, 42, 298–305. [Google Scholar] [CrossRef]
- Begue, B.; Wajant, H.; Bambou, J.; Dubuquoy, L.; Siegmund, D.; Beaulieu, J.; Canioni, D.; Berrebi, D.; Brousse, N.; Desreumaux, P.; et al. Implication of TNF-Related Apoptosis-Inducing Ligand in Inflammatory Intestinal Epithelial Lesions. Gastroenterology 2006, 130, 1962–1974. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Feng, H.; Guo, S.; Han, Y.; Chen, X. Danshenol A inhibits TNF-α-induced expression of intercellular adhesion molecule-1 (ICAM-1) mediated by NOX4 in endothelial cells. Sci. Rep. 2017, 7, 12953. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Rathor, R.; Meena, D.K.; Shyam, R.; Misra, K. Immunostimulatory Activity Investigation of Aqueous and Hydroethanolic Extract of Wheatgrass Using THP1 Cells. MOJ Immunol. 2017, 5, 00146. [Google Scholar] [CrossRef]
- Håversen, L.; Danielsson, K.N.; Fogelstrand, L.; Wiklund, O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis 2009, 202, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-C.; Hsieh, C.-C.; Lin, W.-C. Characterization and immunomodulatory activity of rice hull polysaccharides. Carbohydr. Polym. 2015, 124, 150–156. [Google Scholar] [CrossRef]
- Hou, H.; Fan, Y.; Li, B.; Xue, C.; Yu, G. Preparation of immunomodulatory hydrolysates from Alaska pollock frame. J. Sci. Food Agric. 2012, 92, 3029–3038. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yang, L.; Wang, C.; Ma, S.; Cui, L.; Huang, S.; Sheng, X.; Weng, Q.; Xu, M. Immunostimulatory Activity of Protein Hydrolysate from Oviductus Ranae on Macrophage In Vitro. Evid.-Based Complement. Altern. Med. 2014, 2014, 180234. [Google Scholar] [CrossRef]
- Yang, Q.; Cai, X.; Huang, M.; Wang, S. A specific peptide with immunomodulatory activity from Pseudostellaria heterophylla and the action mechanism. J. Funct. Foods 2020, 68, 103887. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Z.; Pei, X.; Han, X.; Wang, J.; Wang, L.; Long, Z.; Shen, X.; Li, Y. Immunomodulatory effects of marine oligopeptide preparation from Chum Salmon (Oncorhynchus keta) in mice. Food Chem. 2009, 113, 464–470. [Google Scholar] [CrossRef]
- Duarte, J.; Vinderola, G.; Ritz, B.; Perdigón, G.; Matar, C. Immunomodulating capacity of commercial fish protein hydrolysate for diet supplementation. Immunobiology 2006, 211, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammatory Mechanisms: The Molecular Basis of Inflammation and Disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.-B.; Je, J.-Y.; Cho, Y.-S. Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis. Food Res. Int. 2012, 49, 92–98. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cha, H.-J.; Lee, H.; Hong, S.-H.; Park, C.; Park, S.-H.; Kim, G.-Y.; Kim, S.; Kim, H.-S.; Hwang, H.-J.; et al. Protective Effect of Glutathione against Oxidative Stress-induced Cytotoxicity in RAW 264.7 Macrophages through Activating the Nuclear Factor Erythroid 2-Related Factor-2/Heme Oxygenase-1 Pathway. Antioxidants 2019, 8, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrochano, A.R.; Ferraretto, A.; Arranz, E.; Stuknytė, M.; Bottani, M.; O’Connor, P.M.; Giblin, L. Bovine whey peptides transit the intestinal barrier to reduce oxidative stress in muscle cells. Food Chem. 2019, 288, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| BW-SPH-A | BW-SPH-B | BW-SPH-C | BW-SPH-D | BW-SPH-E | BW-SPH-F | |
|---|---|---|---|---|---|---|
| IC50 value (mg dw/mL) | 2.10 ± 0.12 | 2.31 ± 0.13 | 2.14 ± 0.22 | 2.34 ± 0.30 | 2.11 ± 0.15 | 2.47 ± 0.04 |
| Sample Code | ORAC Value (μmol TE/g dw) | FRAP Value (μmol TE/g dw) |
|---|---|---|
| BW-SPH-A | 387.65 ± 9.97 ab | 7.41 ± 0.15 bc |
| BW-SPH-A-GI | 459.73 ± 8.72 A* | 5.60 ± 0.03 A* |
| BW-SPH-B | 350.65 ± 10.35 bc | 7.25 ± 0.22 c |
| BW-SPH-B-GI | 414.20 ± 4.68 * | 5.06 ± 0.04 B* |
| BW-SPH-C | 330.79 ± 9.76 c | 7.67 ± 0.14 bc |
| BW-SPH-C-GI | 348.49 ± 4.89 C | 4.64 ± 0.09 C* |
| BW-SPH-D | 393.32 ± 3.32 a | 8.45 ± 0.08 a |
| BW-SPH-D-GI | 409.00 ± 2.98 B* | 4.75 ± 0.06 BC* |
| BW-SPH-E | 365.88 ± 8.27 abc | 7.57 ± 0.10 bc |
| BW-SPH-E-GI | 386.50 ± 3.23 B | 4.74 ± 0.09 BC* |
| BW-SPH-F | 345.78 ± 4.26 c | 8.03 ± 0.23 ab |
| BW-SPH-F-GI | 385.01 ± 9.55 B* | 4.48 ± 0.08 C* |
| Sample Code (0.5% w/v dw) | GSH Concentration (% tBOOH) | CAT Activity (% H2O2) | ROS Production (% H2O2) |
|---|---|---|---|
| Control | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| BW-SPH-A | 140.3 ± 14.1 * | 137.6 ± 7.4 ** | 86.4 ± 1.8 * |
| BW-SPH-A-GI | 138.5 ± 12.7 * | 133.2 ± 4.6 | 81.0 ± 3.7 * |
| BW-SPH-B | 79.7 ± 11.1 | 123 ± 1.2 | 89.9 ± 2.1 |
| BW-SPH-B-GI | 124.8 ± 14.4 # | 97.8 ± 4.2 | 90.7 ± 5.7 |
| BW-SPH-C | 102.3 ± 8.0 | 125.7 ± 3.1 | 89.7 ± 3.2 |
| BW-SPH-C-GI | 94.3 ± 13.2 | 138.3 ± 16.9 | 90.2 ± 3.0 |
| BW-SPH-D | 82.5 ± 12.2 | 125.6 ± 9.1 | 92.4 ± 3.0 |
| BW-SPH-D-GI | 108.7 ± 13.8 | 116.6 ± 9.3 | 104.3 ± 7.6 |
| BW-SPH-E | 108.6 ± 8.8 | 110.6 ± 11.6 | 94.7 ± 7.3 |
| BW-SPH-E-GI | 113.0 ± 9.4 | 146.4 ± 11.4 * | 91.7 ± 3.5 |
| BW-SPH-F | 97.2 ± 13.4 | 128.4 ± 10.4 | 104.1 ± 6.5 |
| BW-SPH-F-GI | 113.7 ± 4.5 | 128.5 ± 13.8 | 96.1 ± 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heffernan, S.; Harnedy-Rothwell, P.A.; Gite, S.; Whooley, J.; Giblin, L.; Fitzgerald, R.J.; O’Brien, N.M. Blue Whiting Protein Hydrolysates Exhibit Antioxidant and Immunomodulatory Activities in Stimulated Murine RAW264.7 Cells. Appl. Sci. 2021, 11, 9762. https://doi.org/10.3390/app11209762
Heffernan S, Harnedy-Rothwell PA, Gite S, Whooley J, Giblin L, Fitzgerald RJ, O’Brien NM. Blue Whiting Protein Hydrolysates Exhibit Antioxidant and Immunomodulatory Activities in Stimulated Murine RAW264.7 Cells. Applied Sciences. 2021; 11(20):9762. https://doi.org/10.3390/app11209762
Chicago/Turabian StyleHeffernan, Shauna, Pádraigín A. Harnedy-Rothwell, Snehal Gite, Jason Whooley, Linda Giblin, Richard J. Fitzgerald, and Nora M. O’Brien. 2021. "Blue Whiting Protein Hydrolysates Exhibit Antioxidant and Immunomodulatory Activities in Stimulated Murine RAW264.7 Cells" Applied Sciences 11, no. 20: 9762. https://doi.org/10.3390/app11209762
APA StyleHeffernan, S., Harnedy-Rothwell, P. A., Gite, S., Whooley, J., Giblin, L., Fitzgerald, R. J., & O’Brien, N. M. (2021). Blue Whiting Protein Hydrolysates Exhibit Antioxidant and Immunomodulatory Activities in Stimulated Murine RAW264.7 Cells. Applied Sciences, 11(20), 9762. https://doi.org/10.3390/app11209762