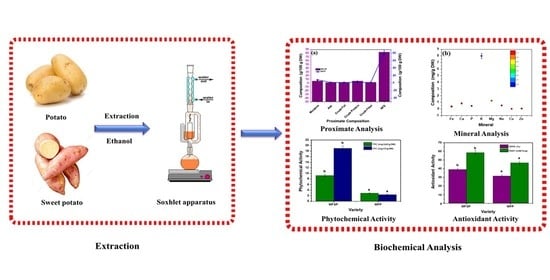
Comparative Study of Potato (Solanum tuberosum L.) and Sweet Potato (Ipomoea batatas L.): Evaluation of Proximate Composition, Polyphenol Content, Mineral and Antioxidant Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Physiochemical Parameters Analysis
2.3.1. Proximate, Colour and Mineral Analysis
2.3.2. Phytochemical Analysis
2.4. In Vitro Antioxidant Analysis
2.4.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition Analysis
3.2. Colour Analysis
3.3. Mineral’s Analysis
3.4. Phytochemical Analysis
3.4.1. Total Phenolic Content in WFSP and WFP
3.4.2. Total Flavonoids Content in WFSP and WFP
3.5. Antioxidant Activity
3.5.1. DPPH Assay Analysis
3.5.2. FRAP Assay Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forgie, A.J.; Drall, K.M.; Bourque, S.L.; Field, C.J.; Kozyrskyj, A.L.; Willing, B.P. The impact of maternal and early life malnutrition on health: A diet-microbe perspective. BMC Med. 2020, 18, 135. [Google Scholar] [CrossRef]
- Cohen, K.; Weinstein, A.M. Synthetic and non-synthetic cannabinoid drugs and their adverse effects—A review from public health prospective. Front. Public Health 2018, 6, 162. [Google Scholar] [CrossRef]
- Morley, C. Critical Perspectives in Clinical Nutrition Practice. In Critical Dietetics and Critical Nutrition Studies; Springer: Berlin, Germany, 2019; pp. 69–83. [Google Scholar]
- Williamson, C. Functional foods: What are the benefits? Br. J. Community Nurs. 2009, 14, 230–236. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.; Fan, M.; Yu, R.; Zhang, Y.; Liu, J.; Zhou, X.; Cai, Y.; Huang, S.; Hu, Z. Sodium butyrate inhibits migration and induces AMPK-mTOR pathway-dependent autophagy and ROS-mediated apoptosis via the miR-139-5p/Bmi-1 axis in human bladder cancer cells. FASEB J. 2020, 34, 4266–4282. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Hu, S.; Jin, Q.; Li, D.; Tian, F.; Toan, S.; Li, Y.; Zhou, H.; Chen, Y. Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: A mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol. 2018, 16, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mu, B.; Song, Z.; Ma, Z.; Mu, T. The in vitro antioxidant activity and inhibition of intracellular reactive oxygen species of sweet potato leaf polyphenols. Oxidative Med. Cell. Longev. 2018, 2018, 9017828. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Suleman, M.; Khan, A.; Baqi, A.; Kakar, M.S.; Ayub, M. 2. Antioxidants, its role in preventing free radicals and infectious diseases in human body. Pure Appl. Biol. 2019, 8, 380–388. [Google Scholar] [CrossRef]
- Echegaray, N.; Gómez, B.; Barba, F.J.; Franco, D.; Estévez, M.; Carballo, J.; Marszałek, K.; Lorenzo, J.M. Chestnuts and by-products as source of natural antioxidants in meat and meat products: A review. Trends Food Sci. Technol. 2018, 82, 110–121. [Google Scholar] [CrossRef]
- Mussoline, W.A.; Wilkie, A.C. Feed and fuel: The dual-purpose advantage of an industrial sweetpotato. J. Sci. Food Agric. 2017, 97, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Coulibali, Z.; Cambouris, A.N.; Parent, S.-É. Cultivar-specific nutritional status of potato (Solanum tuberosum L.) crops. PLoS ONE 2020, 15, e0230458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, J.R.; Yao, L.; Charkowski, A.O.; Heuberger, A.L. Metabolites from Wild Potato Inhibit Virulence Factors of the Soft Rot and Blackleg Pathogen Pectobacterium brasiliense. Mol. Plant-Microbe Interact. 2021, 34, 100–109. [Google Scholar] [CrossRef]
- Rose, I.M.; Vasanthakaalam, H. Comparison of the nutrient composition of four sweet potato varieties cultivated in Rwanda. Am. J. Food Nutr. 2011, 1, 34–38. [Google Scholar] [CrossRef]
- FAO. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 10 May 2021).
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.-U.; Jahan, N.; Jabeen, Z. The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S.; Daramy, M.A. Nutrients, minerals, antioxidant pigments and phytochemicals, and antioxidant capacity of the leaves of stem amaranth. Sci. Rep. 2020, 10, 3892. [Google Scholar] [CrossRef] [Green Version]
- Batool, A.; Hazafa, A.; Ahmad, S.; Khan, H.A.; Abideen, H.M.; Zafar, A.; Bilal, M.; Iqbal, H.M. Treatment of lymphomas via regulating the Signal transduction pathways by natural therapeutic approaches: A review. Leuk. Res. 2021, 104, 106554. [Google Scholar] [CrossRef]
- Jokioja, J.; Linderborg, K.M.; Kortesniemi, M.; Nuora, A.; Heinonen, J.; Sainio, T.; Viitanen, M.; Kallio, H.; Yang, B. Anthocyanin-rich extract from purple potatoes decreases postprandial glycemic response and affects inflammation markers in healthy men. Food Chem. 2020, 310, 125797. [Google Scholar] [CrossRef]
- Teow, C.C.; Truong, V.-D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Rumbaoa, R.G.O.; Cornago, D.F.; Geronimo, I.M. Phenolic content and antioxidant capacity of Philippine sweet potato (Ipomoea batatas) varieties. Food Chem. 2009, 113, 1133–1138. [Google Scholar] [CrossRef]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) Lam.). Sci. Rep. 2018, 8, 1–12. [Google Scholar]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of Aoac International, 18th Edgaithersburg; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Omoba, O.S.; Oyewole, G.O.; Oloniyo, R.O. Chemical Compositions and Antioxidant Properties of Orange Fleshed Sweet Potato Leaves and the Consumer Acceptability in Vegetable Soup. Prev. Nutr. Food Sci. 2020, 25, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K.; Sams, S.; Rana, Z.H.; Akhtaruzzaman, M.; Islam, S.N. Minerals, vitamin C, and effect of thermal processing on carotenoids composition in nine varieties orange-fleshed sweet potato (Ipomoea batatas L.). J. Food Compos. Anal. 2020, 92, 103582. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Talei, D.; Jaafar, H.Z.; Juraimi, A.S.; Mohamed, M.T.M.; Puteh, A.; Halim, M.R.A. Plant-growth regulators alter phytochemical constituents and pharmaceutical quality in Sweet potato (Ipomoea batatas L.). BMC Complement. Altern. Med. 2016, 16, 152. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Mu, T.; Sun, H. Profiling of phenolic acids and flavonoids in sweet potato (Ipomoea batatas L.) leaves and evaluation of their anti-oxidant and hypoglycemic activities. Food Biosci. 2021, 39, 100801. [Google Scholar] [CrossRef]
- Liu, J.; Mu, T.; Sun, H.; Fauconnier, M.L. Optimization of ultrasonic–microwave synergistic extraction of flavonoids from sweet potato leaves by response surface methodology. J. Food Process. Preserv. 2019, 43, e13928. [Google Scholar] [CrossRef]
- Kourouma, V.; Mu, T.-H.; Zhang, M.; Sun, H.-N. Effects of cooking process on carotenoids and antioxidant activity of orange-fleshed sweet potato. Lwt 2019, 104, 134–141. [Google Scholar] [CrossRef]
- Alam, M.K.; Rana, Z.H.; Islam, S.N. Comparison of the proximate composition, total carotenoids and total polyphenol content of nine orange-fleshed sweet potato varieties grown in Bangladesh. Foods 2016, 5, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. Ultrasound-assisted extraction of polyphenols from potato peels: Profiling and kinetic modelling. Int. J. Food Sci. Technol. 2017, 52, 1432–1439. [Google Scholar] [CrossRef] [Green Version]
- Kourouma, V.; Mu, T.H.; Zhang, M.; Sun, H.N. Comparative study on chemical composition, polyphenols, flavonoids, carotenoids and antioxidant activities of various cultivars of sweet potato. Int. J. Food Sci. Technol. 2020, 55, 369–378. [Google Scholar] [CrossRef]
- Moura, I.O.; Santana, C.C.; Lourenço, Y.R.F.; Souza, M.F.; Silva, A.R.S.T.; Dolabella, S.S.; e Silva, A.M.d.O.; Oliveira, T.B.; Duarte, M.C.; Faraoni, A.S. Chemical Characterization, Antioxidant Activity and Cytotoxicity of the Unconventional Food Plants: Sweet Potato (Ipomoea batatas (L.) Lam.) Leaf, Major Gomes (Talinum paniculatum (Jacq.) Gaertn.) and Caruru (Amaranthus deflexus L.). Waste Biomass Valorization 2021, 12, 2407–2431. [Google Scholar] [CrossRef]
- Sołtys-Kalina, D.; Murawska, Z.; Strzelczyk-Żyta, D.; Wasilewicz-Flis, I.; Marczewski, W. Phytotoxic potential of cultivated and wild potato species (Solanum sp.): Role of glycoalkaloids, phenolics and flavonoids in phytotoxicity against mustard (Sinapis alba L.). Acta Physiol. Plant. 2019, 41, 55. [Google Scholar] [CrossRef] [Green Version]
- Galani, J.H.Y.; Mankad, P.M.; Shah, A.K.; Patel, N.J.; Acharya, R.R.; Talati, J.G. Effect of storage temperature on vitamin C, total phenolics, UPLC phenolic acid profile and antioxidant capacity of eleven potato (Solanum tuberosum) varieties. Hortic. Plant J. 2017, 3, 73–89. [Google Scholar] [CrossRef]
- Friedman, M.; Kozukue, N.; Kim, H.-J.; Choi, S.-H.; Mizuno, M. Glycoalkaloid, phenolic, and flavonoid content and antioxidative activities of conventional nonorganic and organic potato peel powders from commercial gold, red, and Russet potatoes. J. Food Compos. Anal. 2017, 62, 69–75. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, B.W.; Lee, H.U.; Lee, Y.Y.; Kim, M.H.; Lee, J.Y.; Lee, B.K.; Woo, K.S.; Kim, H.J. Phenolic compounds and antioxidant activity in sweet potato after heat treatment. J. Sci. Food Agric. 2019, 99, 6833–6840. [Google Scholar] [CrossRef]
- Oloniyo, R.O.; Omoba, O.S.; Awolu, O.O.; Olagunju, A.I. Orange-fleshed sweet potatoes composite bread: A good carrier of beta (β)-carotene and antioxidant properties. J. Food 2021, 45, e13423. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshad, A.; Iqbal, H.; Siddiqa, A.; Zulfiqar, T.; Tareen, M.B.K.; Amna, D.; Shakir, M.; Hazafa, A.; Naeem, M.; Lorenzo, J.M.; et al. Comparative Study of Potato (Solanum tuberosum L.) and Sweet Potato (Ipomoea batatas L.): Evaluation of Proximate Composition, Polyphenol Content, Mineral and Antioxidant Activities. Appl. Sci. 2021, 11, 11844. https://doi.org/10.3390/app112411844
Arshad A, Iqbal H, Siddiqa A, Zulfiqar T, Tareen MBK, Amna D, Shakir M, Hazafa A, Naeem M, Lorenzo JM, et al. Comparative Study of Potato (Solanum tuberosum L.) and Sweet Potato (Ipomoea batatas L.): Evaluation of Proximate Composition, Polyphenol Content, Mineral and Antioxidant Activities. Applied Sciences. 2021; 11(24):11844. https://doi.org/10.3390/app112411844
Chicago/Turabian StyleArshad, Ammara, Hira Iqbal, Ayesha Siddiqa, Taha Zulfiqar, Muhammad B. K. Tareen, Dua Amna, Muhammad Shakir, Abu Hazafa, Muhammad Naeem, José M. Lorenzo, and et al. 2021. "Comparative Study of Potato (Solanum tuberosum L.) and Sweet Potato (Ipomoea batatas L.): Evaluation of Proximate Composition, Polyphenol Content, Mineral and Antioxidant Activities" Applied Sciences 11, no. 24: 11844. https://doi.org/10.3390/app112411844
APA StyleArshad, A., Iqbal, H., Siddiqa, A., Zulfiqar, T., Tareen, M. B. K., Amna, D., Shakir, M., Hazafa, A., Naeem, M., Lorenzo, J. M., & Domínguez, R. (2021). Comparative Study of Potato (Solanum tuberosum L.) and Sweet Potato (Ipomoea batatas L.): Evaluation of Proximate Composition, Polyphenol Content, Mineral and Antioxidant Activities. Applied Sciences, 11(24), 11844. https://doi.org/10.3390/app112411844