Quality Evaluation of Plant Oil Blends Interesterified by Using Immobilized Rhizomucor miehei Lipase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
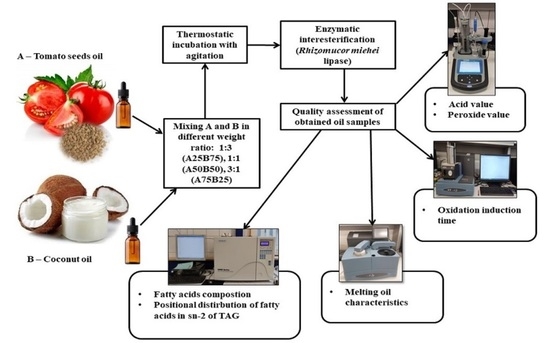
2.2. Preparation of the Enzymatic Interesterification
2.3. Analytical Methods
2.3.1. Determination of Fatty Acids Profile
2.3.2. Determination of Fatty Acids Positional Distribution in Triacylglycerols
2.3.3. Determination of Oxidative Stability
2.3.4. Determination of Melting Characteristics
2.3.5. Determination of Acid Value
2.3.6. Determination of Peroxide Value
2.4. Statistical Analysis
3. Results
3.1. Fatty Acids Profile
3.2. Fatty Acids Distribution in Triacylglycerol Structure
3.3. Oxidative Stability
3.4. Melting Characteristics
3.5. Acid Value of Interesterification Products
3.6. Peroxide Value of Interesterification Products
4. Discussion
4.1. Fatty Acids Profile
4.2. Fatty Acids Distribution in Triacylglycerol Structure
4.3. Oxidative Stability
4.4. Melting Characteristics
4.5. Acid Value of Interesterification Products
4.6. Peroxide Value of Interesterification Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Achremowicz, K.; Szary-Sworst, K. Wielonienasycone kwasy tłuszczowe czynnikiem poprawy stanu zdrowia człowieka. Żywność Nauka Technol. Jakość 2005, 12, 23–35. [Google Scholar]
- Mazzocchi, A.; De Cosmi, V.; Risé, P.; Milani, G.P.; Turolo, S.; Syrén, M.L.; Agostoni, C. Bioactive compounds in edible oils and their role in oxidative stress and inflammation. Front. Physiol. 2021, 12, 659551. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Ruiz, J.C.; Ortiz Vazquez ED, L.L.; Segura Campos, M.R. Encapsulation of vegetable oils as source of omega-3 fatty acids for enriched functional foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 1423–1434. [Google Scholar] [CrossRef]
- Nagy, K.; Tiuca, I. Importance of Fatty Acids in Physiopathology of Human Body. In Fatty Acids; IntechOpen: London, UK, 2017. [Google Scholar]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Roopashree, P.G.; Shetty, S.S.; Kumari, N.S. Effect of medium chain fatty acid in human health and disease. J. Funct. Foods 2021, 87, 104724. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Hu, J.N.; Zhu, X.M.; Bai, C.Q.; Peng, H.L.; Xiong, H.; Hu, J.W.; Zhao, Q. Characteristics and feasibility of trans-free plastic fats through lipozyme TL IM-catalyzed interesterification of palm stearin and akebia trifoliata variety Australis seed oil. J. Agric. Food Chem. 2014, 62, 3293–3300. [Google Scholar] [CrossRef]
- Nazir, M.; Arif, S.; Khan, R.S.; Nazir, W.; Khalid, N.; Maqsood, S. Opportunities and challenges for functional and medicinal beverages: Current and future trends. Trends Food Sci. Technol. 2019, 88, 513–526. [Google Scholar] [CrossRef]
- Pearce, D.W.; Turner, R.K.; Turner, R.K. Economics of Natural Resources and the Environment; Johns Hopkins University Press: Baltimore, MD, USA, 1990. [Google Scholar]
- Stahel, W.R. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef] [Green Version]
- Al-Obadi, M.; Ayad, H.; Pokharel, S.; Ayari, M.A. Perspectives on food waste management: Prevention and social innovations. Sustain. Prod. Consum. 2022, 31, 190–208. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M. Physicochemical composition of tomato seed oil for an edible use: The effect of cultivar. Int. Food Res. J. 2016, 23, 583–591. [Google Scholar]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Chen, J.; Bi, Y.; Chen, S.; Tan, D.; Peng, D. Effect of lard quality on chemical Interesterification catalyzed by KOH/glycerol. J. Am. Oil Chem. Soc. 2015, 92, 513–521. [Google Scholar] [CrossRef]
- Farfán, M.; Villalón, M.J.; Ortíz, M.E.; Nieto, S.; Bouchon, P. The effect of interesterification on the bioavailability of fatty acids in structured lipids. Food Chem. 2013, 139, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Rohm, H.; Schäper, C.; Zahn, S. Interesterified fats in chocolate and bakery products: A concise review. LWT 2018, 87, 379–384. [Google Scholar] [CrossRef]
- Sharma, M.; Lokesh, B.R. Effect of enzymatic trans-and interesterification on the thermal properties of groundnut and linseed oils and their blends. J. Am. Oil Chem. Soc. 2012, 89, 805–813. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, W.J.; Wang, Y. Evaluation of enzymatic interesterification in structured triacylglycerols preparation: A concise review and prospect. Crit. Rev. Food Sci. Nutr. 2021, 61, 3145–3159. [Google Scholar] [CrossRef]
- Idris, N.A.; Dian, N.L.H.M. Interesterified palm products as alternatives to hydrogenation. Asia Pac. J. Clin. Nutr. 2005, 14, 396. [Google Scholar]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Vegetable oil blending: A review of physicochemical, nutritional and health effects. Trends Food Sci. Technol. 2016, 57, 52–58. [Google Scholar] [CrossRef]
- Iqbal, M.P. Trans fatty acids–A risk factor for cardiovascular disease. Pak. J. Med. Sci. 2014, 30, 194. [Google Scholar] [CrossRef]
- Wirkowska-Wojdyła, M.; Bryś, J.; Górska, A.; Ostrowska-Ligęza, E. Effect of enzymatic interesterification on physiochemical and thermal properties of fat used in cookies. LWT 2016, 74, 99–105. [Google Scholar] [CrossRef]
- Costales-Rodríguez, R.; Gibon, V.; Verhé, R.; De Greyt, W. Chemical and enzymatic interesterification of a blend of palm stearin: Soybean oil for low trans-margarine formulation. J. Am. Oil Chem. Soc. 2009, 86, 681–697. [Google Scholar] [CrossRef]
- Gibon, V. Enzymatic interesterification of oils. Lipid Technol. 2011, 23, 274–277. [Google Scholar] [CrossRef]
- Bryś, J.; Wirkowska, M.; Górska, A.; Ostrowska-Ligęza, E.; Bryś, A.; Koczoń, P. The use of DSC and FT-IR spectroscopy for evaluation of oxidative stability of interesterified fats. J. Therm. Anal. Calorim. 2013, 112, 481–487. [Google Scholar] [CrossRef] [Green Version]
- PN EN ISO 5509:2001; Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. Polish Committee for Standardization: Warsaw, Poland, 2001.
- Bryś, J.; Vaz Flores, I.F.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, A. Use of GC and PDSC methods to characterize human milk fat substitutes obtained from lard and milk thistle oil mixtures. J. Therm. Anal. Calorim. 2017, 130, 319–327. [Google Scholar] [CrossRef]
- Yoshida, H.; Tomiyama, Y.; Yoshida, N.; Shibata, K.; Mizushina, Y. Regiospecific profiles of fatty acids in triacylglycerols and phospholipids from adzuki beans (Vigna angularis). Nutrients 2010, 2, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Tomiyama, Y.; Mizushina, Y. Characterization in the fatty acid distributions of triacyloglicerols and phospholipids in kidney beans (Phaseolus vulgaris L.). J. Food Lipids 2005, 12, 169–180. [Google Scholar] [CrossRef]
- Brzezińska, R.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, J. Thermal and Kinetic Properties of Brazilian Coffea Arabica Beans. Appl. Sci. 2021, 11, 6324. [Google Scholar] [CrossRef]
- Wirkowska, M.; Ostrowska-Ligęza, E.; Górska, A.; Koczoń, P. Thermal properties of fats extracted from powdered baby formulas. J. Therm. Anal. Calorim. 2012, 110, 137–143. [Google Scholar] [CrossRef]
- Polish Standard PN-EN ISO 660:2010; Oleje i Tłuszcze Roślinne Oraz Zwierzęce. Oznaczanie Liczby Kwasowej i Kwasowości. Polish Committee for Standardization: Warsaw, Poland, 2010.
- Polish Standard PN-EN ISO 3960:2012; Oleje i Tłuszcze Roślinne Oraz Zwierzęce. Oznaczanie Liczby Nadtlenkowej. Jodometryczne (Wizualne) Oznaczanie Punktu Końcowego. Polish Committee for Standardization: Warsaw, Poland, 2012.
- Zuorro, A.; Lavecchia, R.; Medici, F. Use of Cell Wall Degrading Enzymes for the Production of High-Quality Functional Products from Tomato Processing Waste. Chem. Eng. Trans. 2014, 38, 355–360. [Google Scholar]
- Giannelos, P.N.; Sxizas, S.; Lois, E.; Zannikos, F.; Anastopoulos, G. Physical, chemical and fuel related properties of tomato seed oil for evaluating its direct use in diesel engines. Ind. Crops Prod. 2005, 22, 193–199. [Google Scholar] [CrossRef]
- Marikkar, J.M.N.; Saraf, D.; Dzulkifly, M.H. Effect of Fractional Crystallyzation on Composition and Thermal Behavior of Coconut Oil. Int. J. Food Prop. 2013, 16, 1284–1292. [Google Scholar] [CrossRef]
- De Avezado, W.M.; De Oliveira, L.F.R.; Alcantara, M.A.; De Magalhes Cordeiro, A.M.T.; De Assis, C.F.; Silva Chaves Damasceno, K.S.F.; Araujo, K.N.; Assis, C.F.; Sousa Junior, F.C. Physicochemical characterization, fatty acid profile, antioxidant activity and antibacterial potential of cacay oil, coconut oil and cacay butter. PLoS ONE 2020, 15, e0232224. [Google Scholar] [CrossRef]
- Bryś, J.; Wirkowska, M. Znaczenie struktury triacylogliceroli w projektowaniu lipidów ustrukturyzowanych. Postępy Tech. Przetwórstwa Spożywczego 2010, 2, 86–89. [Google Scholar]
- Indelicato, S.; Bongiorno, D.; Pitonzo, R.; Stefano, V.; Calabrese, V.; Avellone, G. Triacylglycerols in edible oils: Determination, characterization, quantitation, chemometric approach and evaluation of adulterations. J. Chromatogr. A 2017, 1515, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fabritius, M.; Linderborg, K.M.; Tarvainen, M.; Kalpio, M.; Zhang, Y.; Yang, B. Direct inlet negative ion chemical ionization tandem mass spectrometric analysis of triacylglycerol regioisomers in human milk and infant formulas. Food Chem. 2020, 328, 126991. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, X.; Lee, W.J.; Zhao, G.; Li, C.; Wang, Y. The effects of interesterification on the physicochemical properties of Pangasius bocourti oil and its fractions. Food Chem. 2022, 371, 131177. [Google Scholar] [CrossRef]
- Cordeiro, A.; Medeiros, M.; Silva, M.; Silva, I.; Soledade, L.; Souza, A.; Queiroz, N.; Souza, A.G. Rancimat and PDSC accelerated techniques for evaluation of oxidative stability of soybean oil with plant extracts. J. Agric. Food Chem. 2013, 114, 827–832. [Google Scholar] [CrossRef]
- Kodali, R.D. Oxidative Stability Measurement of High-Stability Oils by Pressure Differential Scanning Calorimeter (PDSC). J. Agric. Food Chem. 2005, 53, 7649–7653. [Google Scholar] [CrossRef]
- Kowalski, B.; Ratusz, K.; Kowalska, D.; Bekas, W. Determination of the oxidative stability of vegetable oils by differential scanning calorimetry and Rancimat measurements. Eur. J. Lipid Sci. Technol. 2004, 106, 165–169. [Google Scholar] [CrossRef]
- Ramezan, Y.; Ghavami, M.; Bahmei, M.; Hadi Givianrad, M.; Hemmasi Hooman, A. The Application of Differential Scanning Calorimetry as a Mean to Determine the Oxidative Stability of Vegetable Oils and its Comparison with Rancimat. Orient. J. Chem. 2015, 31, 1389–1394. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, J.; Lee, W.J.; Akoh, C.C.; Li, A.; Wang, Y. Modification of palm-based oil blend via interesterification: Physicochemical properties, crystallization behaviors and oxidative stabilities. Food Chem. 2021, 347, 129070. [Google Scholar] [CrossRef] [PubMed]
- Maggio, R.M.; Barnaba, C.; Cerretani, L.; Paciulli, M.; Chiavaro, E. Study of the influence of triacylglycerol composition on DSC cooling curves of extra virgin olive oil by chemometric data processing. J. Therm. Anal. Calorim. 2013, 115, 2037–2044. [Google Scholar] [CrossRef]
- Benítez, J.J.; Castillo, P.M.; del Río, J.C.; León-Camacho, M.; Domínguez, E.; Heredia, A.; Guzmán-Puyol, S.; Athanassiou, A.; Heredia-Guerrero, J.A. Valorization of Tomato Processing by-Products: Fatty Acid Extraction and Production of Bio-Based Materials. Materials 2018, 11, 2211. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, Y.; Semwal, A.D.; Sajeevkumar, V.A.; Sharma, G.K. Melting, crystallization and storage stability of virgin coconut oil and its blends by differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR). J. Food Sci. Technol. 2017, 54, 45–54. [Google Scholar] [CrossRef]
- Buczek, B.; Leśniak, A. Analiza właściwości i składu kwasów tłuszczowych handlowych olejów pochodzenia roślinnego. Zesz. Nauk. Uniw. Ekon. W Krakowie 2010, 833, 19–30. [Google Scholar]
- Iskander, M.; Hammam, A.; Sorour, M.; Mehanni, A. Effect of Storage period and antioxidants treatment on physiochemical characteristics and stability of cotton seed and canola oils. J. Environ. Stud. 2009, 1, 55–64. [Google Scholar]
- Ledóchowska, E.; Datta, I. Wpływ frakcji nietriacyloglicerolowej na stabilność oksydatywną tłuszczu przeestryfikowanego chemicznie i enzymatycznie. Żywność 1999, 18, 15–23. [Google Scholar]
- Karim, O.R.; Yangomodou, O.D. Chemical properties of tomato (Lycopersicon escluntum) seed oil. Niger. Food J. 2009, 27. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Tellah, S.; Capocasale, M.; Zappia, C.; Latati, M.; Badiani, M.; Ounane, S.M. Seed oil from ten Algerian peanut landraces for edible use and biodiesel production. J. Oleo Sci. 2016, 65, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Arlee, R.; Suanphairoch, S.; Pakdeechanuan, P. Differences in chemical components and antioxidant-related substances in virgin coconut oil from coconut hybrids and their parents. Int. Food Res. J. 2013, 20, 2103–2109. [Google Scholar]
- Cichosz, G.; Czeczot, H. Stabilność oksydacyjna tłuszczów jadalnych -konsekwencje zdrowotne. Bromatol. I Chem. Toksykol. 2011, 1, 50–60. [Google Scholar]
- Özbek, Z.A.; Çelik, K.; Günç Ergönül, P.; Zeki Hepçimen, A. A Promising Food Waste for Food Fortification: Characterization of Dried Tomato Pomace and Its Cold Pressed Oil. J. Food Chem. Nanotechnol. 2020, 6, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, E.; Aydeniz, B.; Güneşer, O.; Arsunar, E.S. Sensory and physico-chemical properties of cold press-produced tomato (Lycopersicon esculentum L.) seed oils. J. Am. Oil Chem. Soc. 2015, 92, 833–842. [Google Scholar] [CrossRef]
- Subermaniam, L.; Md Saad, Q.H.; Das, S.; Othman, F. Virgin Coconut Oil (VCO) Decreases the Level of Malondialdehyde (MDA) in the Cardiac Tissue of Experimental Sprague-Dawley Rats Fed with Heated Palm Oil. J. Med. Bioeng. 2014, 3, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Marina, A.M.; Che Man, Y.B.; Nazimah, S.A.H.; Amin, I. Chemical Properties of Virgin Coconut Oil. J. Am. Oil Chem. Soc. 2009, 86, 301–307. [Google Scholar] [CrossRef]





| Sample Type | Endothermic Peak 1 | Endothermic Peak 2 | Endothermic Peak 3 | |||
|---|---|---|---|---|---|---|
| Minimum Temperature (°C) | Temperature Range (°C) | Minimum Temperature (°C) | Temperature Range (°C) | Minimum Temperature (°C) | Temperature Range (°C) | |
| A25B75 | 16.1 | −13.1 to 25.5 | – | – | – | – |
| A50B50 | −77.0 | −80.1 to −60.0 | −35.0 | −44.0 to −26.0 | 10.4 | −16.5 to 22.5 |
| A75B25 | −76.7 | −80.0 to −60.2 | −37.0 | –45.5 to −21.3 | −6.0 | −21.3 to 15.0 |
| A | −74.6 | −80.0 to −60.0 | −38.7 | –43.8 to −32.4 | −23.6 | −32.4 to 3.9 |
| B | 24.0 | 6.7 to 30.0 | – | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzezińska, R.; Bryś, J.; Giers, O.; Bryś, A.; Górska, A.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M. Quality Evaluation of Plant Oil Blends Interesterified by Using Immobilized Rhizomucor miehei Lipase. Appl. Sci. 2022, 12, 11148. https://doi.org/10.3390/app122111148
Brzezińska R, Bryś J, Giers O, Bryś A, Górska A, Ostrowska-Ligęza E, Wirkowska-Wojdyła M. Quality Evaluation of Plant Oil Blends Interesterified by Using Immobilized Rhizomucor miehei Lipase. Applied Sciences. 2022; 12(21):11148. https://doi.org/10.3390/app122111148
Chicago/Turabian StyleBrzezińska, Rita, Joanna Bryś, Olga Giers, Andrzej Bryś, Agata Górska, Ewa Ostrowska-Ligęza, and Magdalena Wirkowska-Wojdyła. 2022. "Quality Evaluation of Plant Oil Blends Interesterified by Using Immobilized Rhizomucor miehei Lipase" Applied Sciences 12, no. 21: 11148. https://doi.org/10.3390/app122111148
APA StyleBrzezińska, R., Bryś, J., Giers, O., Bryś, A., Górska, A., Ostrowska-Ligęza, E., & Wirkowska-Wojdyła, M. (2022). Quality Evaluation of Plant Oil Blends Interesterified by Using Immobilized Rhizomucor miehei Lipase. Applied Sciences, 12(21), 11148. https://doi.org/10.3390/app122111148