Characterization of Cysteine Cathepsin Expression in the Central Nervous System of Aged Wild-Type and Cathepsin-Deficient Mice
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Sampling
2.3. SDS-PAGE, Immunoblotting, and Densitometry
2.4. Cathepsin Activity Assays
2.5. Immunohistochemistry
2.6. Densitometry Analysis and Statistical Evaluations
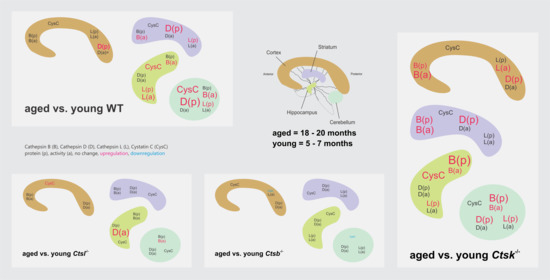
3. Results
3.1. Localization of Cathepsins B, D, and L in Mouse Brain
3.2. Cathepsin B, D and L Protein and Activity Levels in WT and Cathepsin-Deficient Mouse Brain
3.3. Cystatin C Protein Levels in WT and Cathepsin-Deficient Mouse Brain
4. Discussion
4.1. The Proteolytic Network of the Rodent Brain upon Aging
4.2. Potential Contributions of Microglia and Autophagy
4.3. Changes in Wild-Type and Cathepsin-K-Deficient Mouse Brain Tissues upon Aging Differ from Those in Cathepsin B and L Deficiency
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brix, K.; Dunkhorst, A.; Mayer, K.; Jordans, S. Cysteine cathepsins: Cellular roadmap to different functions. Biochimie 2008, 90, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Adair, B.; Reinheckel, T. Specialized roles for cysteine cathepsins in health and disease. J. Clin. Investig. 2010, 120, 3421–3431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadati, T.; Houben, T.; Bitorina, A.; Shiri-Sverdlov, R. The Ins and Outs of Cathepsins: Physiological Function and Role in Disease Management. Cells 2020, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, B.; Tepel, C.; Reinheckel, T.; Deussing, J.; von Figura, K.; Herzog, V.; Peters, C.; Saftig, P.; Brix, K. Thyroid functions of mouse cathepsins B, K, and L. J. Clin. Investig. 2003, 111, 1733–1745. [Google Scholar] [CrossRef] [Green Version]
- Dauth, S.; Arampatzidou, M.; Rehders, M.; Yu, D.; Führer, D.; Brix, K. Thyroid Cathepsin K: Roles in Physiology and Thyroid Disease. Clin. Rev. Bone Miner. Metab. 2011, 9, 94–106. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Oursler, M.J.; Khosla, S. Cathepsin K Inhibitors for Osteoporosis: Biology, Potential Clinical Utility, and Lessons Learned. Endocr. Rev. 2017, 38, 325–350. [Google Scholar] [CrossRef] [Green Version]
- Saftig, P.; Hunziker, E.; Wehmeyer, O.; Jones, S.; Boyde, A.; Rommerskirch, W.; Moritz, J.D.; Schu, P.; von Figura, K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 13453–13458. [Google Scholar] [CrossRef] [Green Version]
- Palermo, C.; Joyce, J.A. Cysteine cathepsin proteases as pharmacological targets in cancer. Trends Pharmacol. Sci. 2008, 29, 22–28. [Google Scholar] [CrossRef]
- Hook, V.; Toneff, T.; Bogyo, M.; Greenbaum, D.; Medzihradszky, K.F.; Neveu, J.; Lane, W.; Hook, G.; Reisine, T. Inhibition of cathepsin B reduces beta-amyloid production in regulated secretory vesicles of neuronal chromaffin cells: Evidence for cathepsin B as a candidate beta-secretase of Alzheimer’s disease. Biol. Chem. 2005, 386, 931–940. [Google Scholar] [CrossRef]
- Hook, V.; Yoon, M.; Mosier, C.; Ito, G.; Podvin, S.; Head, B.P.; Rissman, R.; O’Donoghue, A.J.; Hook, G. Cathepsin B in neurodegeneration of Alzheimer’s disease, traumatic brain injury, and related brain disorders. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140428. [Google Scholar] [CrossRef]
- Nakanishi, H. Microglial cathepsin B as a key driver of inflammatory brain diseases and brain aging. Neural Regen. Res. 2020, 15, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Reinheckel, T.; Deussing, J.; Roth, W.; Peters, C. Towards specific functions of lysosomal cysteine peptidases: Phenotypes of mice deficient for cathepsin B or cathepsin L. Biol. Chem. 2001, 382, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Sevenich, L.; Joyce, J.A. Pericellular proteolysis in cancer. Genes Dev. 2014, 28, 2331–2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoka, V.; Turk, V.; Turk, B. Lysosomal cathepsins and their regulation in aging and neurodegeneration. Ageing Res. Rev. 2016, 32, 22–37. [Google Scholar] [CrossRef]
- Vasiljeva, O.; Reinheckel, T.; Peters, C.; Turk, D.; Turk, V.; Turk, B. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr. Pharm. Des. 2007, 13, 387–403. [Google Scholar] [CrossRef]
- Felbor, U.; Kessler, B.; Mothes, W.; Goebel, H.H.; Ploegh, H.L.; Bronson, R.T.; Olsen, B.R. Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc. Natl. Acad. Sci. USA 2002, 99, 7883–7888. [Google Scholar] [CrossRef] [Green Version]
- Koike, M.; Nakanishi, H.; Saftig, P.; Ezaki, J.; Isahara, K.; Ohsawa, Y.; Schulz-Schaeffer, W.; Watanabe, T.; Waguri, S.; Kametaka, S.; et al. Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons. J. Neurosci. 2000, 20, 6898–6906. [Google Scholar] [CrossRef]
- Dauth, S.; Sîrbulescu, R.F.; Jordans, S.; Rehders, M.; Avena, L.; Oswald, J.; Lerchl, A.; Saftig, P.; Brix, K. Cathepsin K deficiency in mice induces structural and metabolic changes in the central nervous system that are associated with learning and memory deficits. BMC Neurosci. 2011, 12, 74. [Google Scholar] [CrossRef] [Green Version]
- Bednarski, E.; Lynch, G. Cytosolic proteolysis of tau by cathepsin D in hippocampus following suppression of cathepsins B and L. J. Neurochem. 1996, 67, 1846–1855. [Google Scholar] [CrossRef]
- Kenessey, A.; Nacharaju, P.; Ko, L.W.; Yen, S.H. Degradation of tau by lysosomal enzyme cathepsin D: Implication for Alzheimer neurofibrillary degeneration. J. Neurochem. 1997, 69, 2026–2038. [Google Scholar] [CrossRef]
- Mueller-Steiner, S.; Zhou, Y.; Arai, H.; Roberson, E.D.; Sun, B.; Chen, J.; Wang, X.; Yu, G.; Esposito, L.; Mucke, L.; et al. Antiamyloidogenic and neuroprotective functions of cathepsin B: Implications for Alzheimer’s disease. Neuron 2006, 51, 703–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Zhou, Y.; Halabisky, B.; Lo, I.; Cho, S.H.; Mueller-Steiner, S.; Devidze, N.; Wang, X.; Grubb, A.; Gan, L. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer’s disease. Neuron 2008, 60, 247–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Zaitsev, S.; Taylor, G.; d’Azzo, A.; Bonten, E. Protective protein/cathepsin A rescues N-glycosylation defects in neuraminidase-1. Biochim. Biophys. Acta 2009, 1790, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nixon, R.A.; Cataldo, A.M. Lysosomal system pathways: Genes to neurodegeneration in Alzheimer’s disease. J. Alzheimers Dis. 2006, 9, 277–289. [Google Scholar] [CrossRef]
- Vidoni, C.; Follo, C.; Savino, M.; Melone, M.A.; Isidoro, C. The Role of Cathepsin D in the Pathogenesis of Human Neurodegenerative Disorders. Med. Res. Rev. 2016, 36, 845–870. [Google Scholar] [CrossRef]
- Cermak, S.; Kosicek, M.; Mladenovic-Djordjevic, A.; Smiljanic, K.; Kanazir, S.; Hecimovic, S. Loss of Cathepsin B and L Leads to Lysosomal Dysfunction, NPC-Like Cholesterol Sequestration and Accumulation of the Key Alzheimer’s Proteins. PLoS ONE 2016, 11, e0167428. [Google Scholar] [CrossRef] [Green Version]
- Babar, K.M.; Naz, F.; Hassan, A.M.; Nadeem, S.; Nabeela, A.H.; Rabia, M.; Maryam, M.; Amin, A.; Zaira, A.; Zerwa, S.; et al. Role and Molecular Mechanisms of Lysosomes and Cathepsins in Neuropathology and Aging: New Insights. J. Neurodegener. Disord. 2021, 4, 113–121. [Google Scholar]
- Asagiri, M.; Hirai, T.; Kunigami, T.; Kamano, S.; Gober, H.J.; Okamoto, K.; Nishikawa, K.; Latz, E.; Golenbock, D.T.; Aoki, K.; et al. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science 2008, 319, 624–627. [Google Scholar] [CrossRef]
- Sevenich, L.; Pennacchio, L.A.; Peters, C.; Reinheckel, T. Human cathepsin L rescues the neurodegeneration and lethality in cathepsin B/L double-deficient mice. Biol. Chem. 2006, 387, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Deussing, J.; Roth, W.; Saftig, P.; Peters, C.; Ploegh, H.L.; Villadangos, J.A. Cathepsins B and D are dispensable for major histocompatibility complex class II-mediated antigen presentation. Proc. Natl. Acad. Sci. USA 1998, 95, 4516–4521. [Google Scholar] [CrossRef] [Green Version]
- Halangk, W.; Lerch, M.M.; Brandt-Nedelev, B.; Roth, W.; Ruthenbuerger, M.; Reinheckel, T.; Domschke, W.; Lippert, H.; Peters, C.; Deussing, J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J. Clin. Investig. 2000, 106, 773–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, W.; Deussing, J.; Botchkarev, V.A.; Pauly-Evers, M.; Saftig, P.; Hafner, A.; Schmidt, P.; Schmahl, W.; Scherer, J.; Anton-Lamprecht, I.; et al. Cathepsin L deficiency as molecular defect of furless: Hyperproliferation of keratinocytes and pertubation of hair follicle cycling. Faseb J. 2000, 14, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Neuhoff, V.; Philipp, K.; Zimmer, H.G.; Mesecke, S. A simple, versatile, sensitive and volume-independent method for quantitative protein determination which is independent of other external influences. Hoppe Seylers Z Physiol. Chem. 1979, 360, 1657–1670. [Google Scholar] [CrossRef]
- Boda, E.; Pini, A.; Hoxha, E.; Parolisi, R.; Tempia, F. Selection of reference genes for quantitative real-time RT-PCR studies in mouse brain. J. Mol. Neurosci. 2009, 37, 238–253. [Google Scholar] [CrossRef]
- Tanic, N.; Perovic, M.; Mladenovic, A.; Ruzdijic, S.; Kanazir, S. Effects of aging, dietary restriction and glucocorticoid treatment on housekeeping gene expression in rat cortex and hippocampus-evaluation by real time RT-PCR. J. Mol. Neurosci. 2007, 32, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ivy, G.O.; Schottler, F.; Wenzel, J.; Baudry, M.; Lynch, G. Inhibitors of Lysosomal Enzymes: Accumulation of Lipofuscin-Like Dense Bodies in the Brain. Science 1984, 226, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Tominaga, K.; Amano, T.; Hirotsu, I.; Inoue, T.; Yamamoto, K. Age-Related Changes in Activities and Localizations of Cathepsins D, E, B, and L in the Rat Brain Tissues. Exp. Neurol. 1994, 126, 119–128. [Google Scholar] [CrossRef]
- Nixon, R.A.; Cataldo, A.M. The lysosomal system in neuronal cell death: A review. Ann. N. Y. Acad. Sci. 1993, 679, 87–109. [Google Scholar] [CrossRef]
- Qatato, M.; Szumska, J.; Skripnik, V.; Rijntjes, E.; Köhrle, J.; Brix, K. Canonical TSH Regulation of Cathepsin-Mediated Thyroglobulin Processing in the Thyroid Gland of Male Mice Requires Taar1 Expression. Front. Pharmacol. 2018, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Levy, E. Cystatin C in Alzheimer’s disease. Front. Mol. Neurosci. 2012, 5, 79. [Google Scholar] [CrossRef] [Green Version]
- Mathews, P.M.; Levy, E. Cystatin C in aging and in Alzheimer’s disease. Ageing Res. Rev. 2016, 32, 38–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, S.; Song, J.K.; Shin, S.W.; Lee, K.H. Human serum albumin fusion protein as therapeutics for targeting amyloid beta in Alzheimer’s diseases. Neurosci. Lett. 2022, 767, 136298. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Levy, E. Cystatin C: A potential target for Alzheimer’s treatment. Expert Rev. Neurother. 2008, 8, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Werle, B.; Lah, T.; Brunner, N. Cysteine proteinases and their inhibitors in extracellular fluids: Markers for diagnosis and prognosis in cancer. Int. J. Biol. Markers 2000, 15, 84–89. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Nakamura, Y.; Takeda, M.; Suzuki, H.; Morita, H.; Tada, K.; Hariguchi, S.; Nishimura, T. Age-dependent change in activities of lysosomal enzymes in rat brain. Mech. Ageing Dev. 1989, 50, 215–225. [Google Scholar] [CrossRef]
- Banay-Schwartz, M.; DeGuzman, T.; Kenessey, A.; Palkovits, M.; Lajtha, A. The distribution of cathepsin D activity in adult and aging human brain regions. J. Neurochem. 1992, 58, 2207–2211. [Google Scholar] [CrossRef]
- Haas, U.; Sparks, D.L. Cortical cathepsin D activity and immunolocalization in Alzheimer disease, critical coronary artery disease, and aging. Mol. Chem. Neuropathol. 1996, 29, 1–14. [Google Scholar] [CrossRef]
- von Bernhardi, R.; Eugenín-von Bernhardi, L.; Eugenín, J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, H. Microglial functions and proteases. Mol. Neurobiol. 2003, 27, 163–176. [Google Scholar] [CrossRef]
- Nakanishi, H.; Wu, Z. Microglia-aging: Roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav. Brain Res. 2009, 201, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nixon, R.A.; Yang, D.S.; Lee, J.H. Neurodegenerative lysosomal disorders: A continuum from development to late age. Autophagy 2008, 4, 590–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reznick, A.Z.; Gershon, D. The effect of age on the protein degradation system in the nematode Turbatrix aceti. Mech. Ageing Dev. 1979, 11, 403–415. [Google Scholar] [CrossRef]
- Venugopalan, V.; Al-Hashimi, A.; Rehders, M.; Golchert, J.; Reinecke, V.; Homuth, G.; Völker, U.; Manirajah, M.; Touzani, A.; Weber, J.; et al. The Thyroid Hormone Transporter Mct8 Restricts Cathepsin-Mediated Thyroglobulin Processing in Male Mice through Thyroid Auto-Regulatory Mechanisms That Encompass Autophagy. Int. J. Mol. Sci. 2021, 22, 462. [Google Scholar] [CrossRef]
- Kroemer, G.; Jäättelä, M. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer 2005, 5, 886–897. [Google Scholar] [CrossRef]
- Dauth, S.; Rakov, H.; Sîrbulescu, R.F.; Ilieş, I.; Weber, J.; Batbajar Dugershaw, B.; Braun, D.; Rehders, M.; Wirth, E.K.; Führer, D.; et al. Function of Cathepsin K in the Central Nervous System of Male Mice is Independent of Its Role in the Thyroid Gland. Cell. Mol. Neurobiol. 2020, 40, 695–710. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.M.T.; Dauth, S.; Margineanu, M.B.; Snetkova, V.; Rehders, M.; Jordans, S.; Brix, K. Characterization of Cysteine Cathepsin Expression in the Central Nervous System of Aged Wild-Type and Cathepsin-Deficient Mice. Appl. Sci. 2022, 12, 2608. https://doi.org/10.3390/app12052608
Yu DMT, Dauth S, Margineanu MB, Snetkova V, Rehders M, Jordans S, Brix K. Characterization of Cysteine Cathepsin Expression in the Central Nervous System of Aged Wild-Type and Cathepsin-Deficient Mice. Applied Sciences. 2022; 12(5):2608. https://doi.org/10.3390/app12052608
Chicago/Turabian StyleYu, Denise M. T., Stephanie Dauth, Michael B. Margineanu, Valentina Snetkova, Maren Rehders, Silvia Jordans, and Klaudia Brix. 2022. "Characterization of Cysteine Cathepsin Expression in the Central Nervous System of Aged Wild-Type and Cathepsin-Deficient Mice" Applied Sciences 12, no. 5: 2608. https://doi.org/10.3390/app12052608
APA StyleYu, D. M. T., Dauth, S., Margineanu, M. B., Snetkova, V., Rehders, M., Jordans, S., & Brix, K. (2022). Characterization of Cysteine Cathepsin Expression in the Central Nervous System of Aged Wild-Type and Cathepsin-Deficient Mice. Applied Sciences, 12(5), 2608. https://doi.org/10.3390/app12052608