Response Surface Methodology as a Tool for Optimization of Extraction Process of Bioactive Compounds from Spent Coffee Grounds
Abstract
:Featured Application
Abstract
1. Introduction
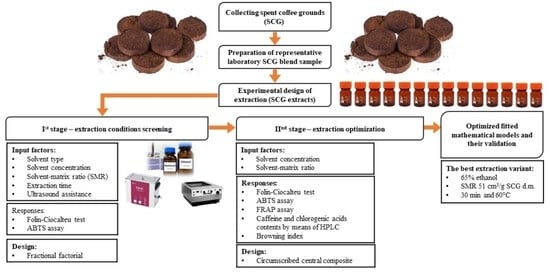
2. Materials and Analytical Methods
2.1. Materials
2.2. Preparation of SCG Extracts
2.3. Statistical Approach in the Experimental Design of Extraction Optimization
2.4. Analytical Methods
2.4.1. Total Polyphenols Content (TPC) Determination
2.4.2. Antioxidant Activity Analysis by ABTS Assay
2.4.3. Antioxidant Activity Analysis by FRAP Assay
2.4.4. High-Performance Liquid Chromatographic (HPLC) Analysis of Caffeine and Chlorogenic Acids
2.4.5. Spectrophotometric Measurements of Browning Index (BI)
3. Results and Discussion
3.1. Determination of the Significant Factors Affecting the Extraction Process
3.2. Optimization of the Extraction Process Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef] [PubMed]
- McNutt, J. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Gosalvitr, P.; Cuellar-Franca, R.; Smith, R.; Azapagic, A. An environmental and economic sustainability assessment of coffee production in the UK. Chem. Eng. J. 2023, 465, 142793. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- International Coffee Organization (ICO). Statistics. Trade Statistics Tables. World Coffee Consumption. Available online: http://www.ico.org/prices/new-consumption-table.pdf (accessed on 10 May 2023).
- International Coffee Organization (ICO). Historical Data on the Global Coffee Trade. Available online: https://www.ico.org/new_historical.asp (accessed on 10 May 2023).
- Wu, C.-T.; Agrawal, D.C.; Huang, W.-Y.; Hsu, H.-C.; Yang, S.-J.; Huang, S.-L.; Lin, Y.-S. Functionality analysis of spent cofee ground extracts obtained by the hydrothermal method. J. Chem. 2019, 2019, 4671438. [Google Scholar] [CrossRef] [Green Version]
- Iriondo-DeHond, A.; Garcia, N.A.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Escobar, F.V.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. 2019, 51, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Winans, K.; Kendall, A.; Deng, H. The history and current applications of the circular economy concept. Renew. Sust. Energ. Rev. 2017, 68, 825–833. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recyl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Tech. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Mesías, M.; Delgado-Andrade, C. Melanoidins as a potential functional food ingredient. Curr. Opin. Food Sci. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Bevilacqua, E.; Cruzat, V.; Singh, I.; Rose’Meyer, R.B.; Panchal, S.K.; Brown, L. The Potential of Spent Coffee Grounds in Functional Food Development. Nutrients 2023, 15, 994. [Google Scholar] [CrossRef] [PubMed]
- Mofijur, M.; Kusumo, F.; Fattah, I.M.R.; Mahmudul, H.M.; Rasul, M.G.; Shamsuddin, A.H.; Mahlia, T.M.I. Resource Recovery from Waste Coffee Grounds Using Ultrasonic-Assisted Technology for Bioenergy Production. Energies 2020, 13, 1770. [Google Scholar] [CrossRef] [Green Version]
- Gigliobianco, M.R.; Campisi, B.; Vargas Peregrina, D.; Censi, R.; Khamitova, G.; Angeloni, S.; Caprioli, G.; Zannotti, M.; Ferraro, S.; Giovannetti, R.; et al. Optimization of the Extraction from Spent Coffee Grounds Using the Desirability Approach. Antioxidants 2020, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Jang, Y.W.; Lee, J.; Kim, S.; Park, C.; Yoo, H.Y. Statistical Optimization of Alkali Pretreatment to Improve Sugars Recovery from Spent Coffee Grounds and Utilization in Lactic Acid Fermentation. Processes 2021, 9, 494. [Google Scholar] [CrossRef]
- Mitraka, G.-C.; Kontogiannopoulos, K.N.; Batsioula, M.; Banias, G.F.; Assimopoulou, A.N. Spent Coffee Grounds’ Valorization towards the Recovery of Caffeine and Chlorogenic Acid: A Response Surface Methodology Approach. Sustainability 2021, 13, 8818. [Google Scholar] [CrossRef]
- Coelho, J.P.; Robalo, M.P.; Boyadzhieva, S.; Stateva, R.P. Microwave-Assisted Extraction of Phenolic Compounds from Spent Coffee Grounds. Process Optimization Applying Design of Experiments. Molecules 2021, 26, 7320. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimized Isolation Procedure for the Extraction of Bioactive Compounds from Spent Coffee Grounds. Appl. Sci. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Balzano, M.; Loizzo, M.R.; Tundis, R.; Lucci, P.; Nunez, O.; Fiorini, D.; Giardinieri, A.; Frega, N.G.; Pacetti, D. Spent espresso coffee grounds as a source of anti-proliferative and antioxidant compounds. Innov. Food Sci. Emerg. 2022, 59, 102254. [Google Scholar] [CrossRef]
- Piasecka, I.; Wiktor, A.; Górska, A. Alternative methods of bioactive compounds and oils extraction from berry fruit by-products—A review. Appl. Sci. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Chmelová, D.; Škulcová, D.; Legerská, B.; Horník, M.; Ondrejovič, M. Ultrasonic-assisted extraction of polyphenols and antioxidants from Picea abies bark. J. Biotech. 2020, 314, 25–33. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Al Jbawi, E.; Saewan, S.A. Traditional and innovative approaches for the extraction of bioactive compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Latha, S.; Sivaranjani, G.; Dhanasekaran, D. Response surface methodology: A non-conventional statistical tool to maximize the throughput of Streptomyces species biomass and their bioactive metabolites. Crit. Rev. Microbiol. 2017, 43, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. In Breakthroughs in Statistics; Kotz, S., Johnson, N.L., Eds.; Springer Series in Statistics; Springer: New York, NY, USA, 1992; pp. 270–310. [Google Scholar]
- Weremfo, A.; Abassah-Oppong, S.; Adulley, F.; Dabie, K.; Seidu-Larry, S. Response surface methodology as a tool to optimize the extraction of bioactive compounds from plant sources. J. Sci. Food Agric. 2023, 103, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Polish Norm: PN-ISO 3534-2:2010; Statistics—Vocabulary and Symbols—Part 2: Applied Statistics. Polish Committee for Standardization: Warsaw, Poland, 2010.
- Polish Norm: PN-ISO 11294:2002; Roasted Ground Coffee—Determination of Moisture Content—Method by Determination of Loss in Mass at 103 Degrees C (Routine Method). Polish Committee for Standardization: Warsaw, Poland, 2002.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Głowacka, R.; Górska, A.; Wirkowska-Wojdyła, M.; Wołosiak, R.; Majewska, E.; Derewiaka, D. The influence of brewing method on bioactive compounds residues in spent coffee grounds of different roasting degree and geographical origin. Int. J. Food Sci. Technol. 2019, 54, 3008–3014. [Google Scholar] [CrossRef]
- Bravo, J.; Juàniz, I.; Monente, C.; Caemmerer, B.; Kroh, L.W.; Paz De Peña, M.; Cid, C. Evaluation of spent coffee obtained from the most common coffeemakers as a source of hydrophilic bioactive compounds. J. Agric. Food Chem. 2012, 60, 12565–12573. [Google Scholar] [CrossRef]
- Brzezińska, R.; Wirkowska-Wojdyła, M.; Piasecka, I.; Górska, A. Application of Response Surface Methodology to Optimize the Extraction Process of Bioactive Compounds Obtained from Coffee Silverskin. Appl. Sci. 2023, 13, 5388. [Google Scholar] [CrossRef]
- Shewale, S.; Rathod, V.K. Extraction of total phenolic content from Azadirachta indica or (neem) leaves: Kinetics study. Prep. Biochem. Biotech. 2018, 48, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Cui, J.; Zhang, H.; Duan, Y.; Zhang, D.; Cai, M.; Chen, G. Ultrasound assisted extraction of polyphenolic compounds from red sorghum (Sorghum bicolor L.) bran and their biological activities and polyphenolic compositions. Ind. Crops Prod. 2018, 112, 296–304. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Cares, M.G.; Vargas, Y.; Gaete, L.; Sainz, J.; Alarcon, J. Ultrasonically assisted extraction of bioactive principles from Quillaja Saponaria Molina. Phys. Procedia 2010, 3, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Oroian, M.; Ursachi, F.; Dranca, F. Ultrasound-assisted extraction of polyphenols from crude pollen. Antioxidants 2020, 9, 322. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.; Monente, C.; Juaniz, I.; Paz De Peña, M.; Cid, C. Influence of extraction process on antioxidant capacity of spent coffee. Food Res. Int. 2013, 50, 610–616. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Selection of the solvent and extraction conditions for maximum recovery of antioxidant phenolic compounds from coffee silverskin. Food Bioprocess Tech. 2014, 7, 1322–1332. [Google Scholar] [CrossRef] [Green Version]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef] [PubMed]
- Carciochi, R.A.; Dieu, V.; Vauchel, P.; Pradal, D.; Dimitrov, K. Reduction of environmental impacts of caffeine extraction from guarana by using ultrasound assistance. Food Bioprod. Proc. 2021, 127, 266–275. [Google Scholar] [CrossRef]
- Tavagnacco, L.; Schnupf, U.; Mason, P.E.; Saboungi, M.-L.; Cesàro, A.; Brady, J.W. Molecular Dynamics Simulation Studies of Caffeine Aggregation in Aqueous Solution. J. Phys. Chem. B 2011, 115, 10957–10966. [Google Scholar] [CrossRef] [Green Version]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of natural antioxidants from spent coffee grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Esquivel, P.; Jimenez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Typek, R. Transformation of chlorogenic acids during the coffee beans roasting process. Eur. Food Res. Technol. 2017, 243, 379–390. [Google Scholar] [CrossRef]
- Del Pino-García, R.; Gonzalez-SanJose, M.L.; Rivero-Perez, M.D.; Muniz, P. Influence of the degree of roasting on the antioxidant capacity and genoprotective effect of instant coffee: Contribution of the melanoidin fraction. J. Agric. Food Chem. 2012, 60, 10530–10539. [Google Scholar] [CrossRef]
- Moreira, A.S.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef]
- Starowicz, M.; Ostaszyk, A.; Zieliński, H. The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples. Foods 2021, 10, 967. [Google Scholar] [CrossRef]



| Run | Organic Solvent Concentration | Extraction Time | SMR | Ultrasound Assistance | Solvent Type |
|---|---|---|---|---|---|
| A | B | C | D | E | |
| 1 | 60 (−) | 30 (−) | 40 (+) | Yes (−) | Ethanol (−) |
| 2 | 60 (−) | 90 (+) | 10 (−) | No (+) | Methanol (+) |
| 3 | 100 (+) | 30 (−) | 10 (−) | Yes (−) | Ethanol (−) |
| 4 | 100 (+) | 90 (+) | 10 (−) | No (+) | Ethanol (−) |
| 5 | 60 (−) | 30 (−) | 10 (−) | Yes (−) | Methanol (+) |
| 6 | 80 (0) | 60 (0) | 25 (0) | Yes (−) | Ethanol (−) |
| 7 | 100 (+) | 30 (−) | 40 (+) | Yes (−) | Methanol (+) |
| 8 | 100 (+) | 90 (+) | 10 (−) | Yes (−) | Methanol (+) |
| 9 | 100 (+) | 90 (+) | 40 (+) | Yes (−) | Ethanol (−) |
| 10 | 60 (−) | 30 (−) | 40 (+) | No (+) | Methanol (+) |
| 11 | 100 (+) | 30 (−) | 40 (+) | No (+) | Ethanol (−) |
| 12 | 60 (−) | 90 (+) | 10 (−) | Yes (−) | Ethanol (−) |
| 13 | 80 (0) | 60 (0) | 25 (0) | Yes (−) | Methanol (+) |
| 14 | 60 (−) | 90 (+) | 40 (+) | No (+) | Ethanol (−) |
| 15 | 80 (0) | 60 (0) | 25 (0) | No (+) | Methanol (+) |
| 16 | 100 (+) | 90 (+) | 40 (+) | No (+) | Methanol (+) |
| 17 | 60 (−) | 30 (−) | 10 (−) | No (+) | Ethanol (−) |
| 18 | 80 (0) | 60 (0) | 25 (0) | No (+) | Ethanol (−) |
| 19 | 60 (−) | 90 (+) | 40 (+) | Yes (−) | Methanol (+) |
| 20 | 100 (+) | 30 (−) | 10 (−) | No (+) | Methanol (+) |
| Run | Organic Solvent Concentration | SMR |
|---|---|---|
| A | B | |
| 1 | 80.00 (1) | 20.00 (−1) |
| 2 | 60.00 (0) | 40.00 (0) |
| 3 | 40.00 (−1) | 20.00 (−1) |
| 4 | 60.00 (0) | 40.00 (0) |
| 5 | 60.00 (0) | 40.00 (0) |
| 6 | 60.00 (0) | 11.71 (−1.414) |
| 7 | 60.00 (0) | 40.00 (0) |
| 8 | 80.00 (1) | 60.00 (1) |
| 9 | 60.00 (0) | 68.28 (1.414) |
| 10 | 40.00 (−1) | 60.00 (1) |
| 11 | 31.71 (−1.414) | 40.00 (0) |
| 12 | 88.28 (1.414) | 40.00 (0) |
| 13 | 60.00 (0) | 40.00 (0) |
| Run | Solvent Concentration | Extraction Time | SMR | Ultrasound Assistance | Solvent Type | ABTS | TPC |
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||
| 1 | 60 (−) | 30 (−) | 40 (+) | Yes (−) | Ethanol (−) | 70.02 ± 4.85 | 39.34 ± 2.69 |
| 2 | 60 (−) | 90 (+) | 10 (−) | No (+) | Methanol (+) | 47.80 ± 3.21 | 17.28 ± 1.18 |
| 3 | 100 (+) | 30 (−) | 10 (−) | Yes (−) | Ethanol (−) | 25.97 ± 1.82 | 20.94 ± 1.63 |
| 4 | 100 (+) | 90 (+) | 10 (−) | No (+) | Ethanol (−) | 35.71 ± 2.45 | 13.54 ± 0.99 |
| 5 | 60 (−) | 30 (−) | 10 (−) | Yes (−) | Methanol (+) | 35.15 ± 2.51 | 18.72 ± 1.27 |
| 6 | 80 (0) | 60 (0) | 25 (0) | Yes (−) | Ethanol (−) | 37.15 ± 2.42 | 23.58 ± 1.52 |
| 7 | 100 (+) | 30 (−) | 40 (+) | Yes (−) | Methanol (+) | 68.45 ± 4.79 | 31.35 ± 2.18 |
| 8 | 100 (+) | 90 (+) | 10 (−) | Yes (−) | Methanol (+) | 21.80 ± 1.45 | 16.26 ± 1.18 |
| 9 | 100 (+) | 90 (+) | 40 (+) | Yes (−) | Ethanol (−) | 62.37 ± 4.28 | 30.13 ± 2.06 |
| 10 | 60 (−) | 30 (−) | 40 (+) | No (+) | Methanol (+) | 101.99 ± 5.95 | 28.03 ± 2.02 |
| 11 | 100 (+) | 30 (−) | 40 (+) | No (+) | Ethanol (−) | 66.15 ± 4.52 | 22.35 ± 1.74 |
| 12 | 60 (−) | 90 (+) | 10 (−) | Yes (−) | Ethanol (−) | 34.01 ± 2.38 | 25.10 ± 1.25 |
| 13 | 80 (0) | 60 (0) | 25 (0) | Yes (−) | Methanol (+) | 55.83 ± 3.23 | 27.71 ± 1.84 |
| 14 | 60 (−) | 90 (+) | 40 (+) | No (+) | Ethanol (−) | 97.16 ± 6.52 | 33.07 ± 2.09 |
| 15 | 80 (0) | 60 (0) | 25 (0) | No (+) | Methanol (+) | 35.88 ± 2.41 | 17.81 ± 1.17 |
| 16 | 100 (+) | 90 (+) | 40 (+) | No (+) | Methanol (+) | 68.68 ± 4.63 | 29.37 ± 1.91 |
| 17 | 60 (−) | 30 (−) | 10 (−) | No (+) | Ethanol (−) | 41.70 ± 2.82 | 15.35 ± 0.92 |
| 18 | 80 (0) | 60 (0) | 25 (0) | No (+) | Ethanol (−) | 79.01 ± 5.55 | 25.69 ± 1.80 |
| 19 | 60 (−) | 90 (+) | 40 (+) | Yes (−) | Methanol (+) | 57.56 ± 3.67 | 34.73 ± 2.25 |
| 20 | 100 (+) | 30 (−) | 10 (−) | No (+) | Methanol (+) | 34.38 ± 2.41 | 12.70 ± 0.99 |
| ABTS | TPC | |
|---|---|---|
| Regression equation | ABTS = 46.40 − 0.32 × A + 1.32 × C + 7.01 × D | TPC = 21.60 − 0.11 × A + 0.45 × C − 2.63 × D |
| Model p-value | 7.1 × 10−6 | 1.5 × 10−7 |
| R2 | 80.23% | 87.84% |
| R2 (adjusted) | 76.53% | 85.56% |
| R2 (predicted) | 72.04% | 81.44% |
| Fitted value (A:60; C:40; D:Yes) | 72.94 | 35.75 |
| Run | Solvent Concentration | SMR | ABTS | FRAP | TPC | Caffeine | 3-CQA | BI |
|---|---|---|---|---|---|---|---|---|
| A | B | |||||||
| 1 | 80.00 (1) | 20.00 (−1) | 43.74 ± 2.19 | 74.74 ± 3.74 | 24.07 ± 1.21 | 3.01 ± 0.16 | 2.99 ± 0.17 | 0.24 ± 0.01 |
| 2 | 60.00 (0) | 40.00 (0) | 70.85 ± 3.46 | 78.02 ± 3.87 | 38.15 ± 1.75 | 4.99 ± 0.19 | 6.99 ± 0.33 | 0.21 ± 0.01 |
| 3 | 40.00 (−1) | 20.00 (−1) | 37.87 ± 1.88 | 55.80 ± 2.81 | 18.90 ± 0.95 | 1.56 ± 0.12 | 2.01 ± 0.12 | 0.08 ± 0.01 |
| 4 | 60.00 (0) | 40.00 (0) | 75.06 ± 3.52 | 71.30 ± 3.52 | 34.95 ± 1.72 | 4.72 ± 0.21 | 7.42 ± 0.35 | 0.22 ± 0.02 |
| 5 | 60.00 (0) | 40.00 (0) | 71.54 ± 3.68 | 67.64 ± 3.37 | 33.21 ± 1.56 | 4.21 ± 0.22 | 7.52 ± 0.38 | 0.18 ± 0.01 |
| 6 | 60.00 (0) | 11.71 (−1.414) | 27.14 ± 2.01 | 53.42 ± 2.64 | 16.83 ± 0.83 | 0.86 ± 0.09 | 2.28 ± 0.11 | 0.17 ± 0.01 |
| 7 | 60.00 (0) | 40.00 (0) | 72.92 ± 3.62 | 75.92 ± 3.79 | 29.17 ± 1.44 | 4.35 ± 0.13 | 7.78 ± 0.41 | 0.19 ± 0.02 |
| 8 | 80.00 (1) | 60.00 (1) | 62.85 ± 3.16 | 91.58 ± 4.51 | 31.70 ± 1.58 | 5.73 ± 0.21 | 5.42 ± 0.24 | 0.22 ± 0.02 |
| 9 | 60.00 (0) | 68.28 (1.414) | 60.77 ± 3.15 | 83.81 ± 4.01 | 37.15 ± 1.84 | 2.52 ± 0.20 | 5.92 ± 0.26 | 0.21 ± 0.01 |
| 10 | 40.00 (−1) | 60.00 (1) | 54.24 ± 2.73 | 69.58 ± 3.51 | 26.64 ± 1.33 | 4.21 ± 0.23 | 3.87 ± 0.23 | 0.17 ± 0.01 |
| 11 | 31.71 (−1.414) | 40.00 (0) | 48.47 ± 2.62 | 63.27 ± 3.14 | 19.91 ± 1.03 | 3.94 ± 0.28 | 3.14 ± 0.17 | 0.15 ± 0.02 |
| 12 | 88.28 (1.414) | 40.00 (0) | 53.19 ± 2.57 | 74.78 ± 3.76 | 28.14 ± 1.41 | 5.02 ± 0.21 | 5.64 ± 0.25 | 0.25 ± 0.03 |
| 13 | 60.00 (0) | 40.00 (0) | 73.87 ± 3.52 | 68.33 ± 3.39 | 33.54 ± 1.72 | 4.11 ± 0.09 | 7.85 ± 0.39 | 0.20 ± 0.02 |
| Tested RSM Response | Model | Simplified Fitted Equation | R2 | Model p-Value | Lack-of-Fit p-Value |
|---|---|---|---|---|---|
| ABTS assay | Reduced quadratic | −105.29 + 3.26 × A + 3.29 × B + 0.03 × A2 − 0.03 × B2 | 98.24% | 4.7 × 10−7 | 0.1386 |
| FRAP assay | Linear | 31.54 + 0.36 × A + 0.46 × B | 83.08% | 1.4 × 10−4 | 0.4908 |
| TPC assay | Reduced quadratic | −43.6 + 1.62 × A + 0.97 × B − 0.01 × A2 − 0.01 × B2 | 89.08% | 6.5 × 10−4 | 0.7185 |
| Caffeine (HPLC) | Quadratic | −10.27 + 0.34 × A + 0.29 × B − 0.003 × A2 − 0.003 × B2 − 0.001 × A × B | 95.69% | 1.2 × 10−4 | 0.1433 |
| 3-CQA (HPLC) | Reduced quadratic | −20.18 + 0.56 × A + 0.43 × B − 0.004 × A2 − 0.005 × B2 | 97.09% | 3.5 × 10−6 | 0.1907 |
| BI (Abs420) | Linear with interactions | −0.13 + 0.005 × A + 0.005 × B − 6.9 × 10−5 × A × B | 87.57% | 2.0 × 10−4 | 0.3521 |
| Used RSM Response | Approximated Value | Real Value | Deviation (%) |
|---|---|---|---|
| ABTS (mg Trolox/g SCG d.m.) | 74.49 | 73.04 | −1.9% |
| FRAP (µmol Fe(II)/g SCG d.m.) | 78.00 | 80.76 | 3.5% |
| TPC (mg GAE/g SCG d.m.) | 36.13 | 37.88 | 4.8% |
| Caffeine (mg/g SCG d.m.) | 4.86 | 4.82 | −0.8% |
| 3-CQA (mg/g SCG d.m.) | 7.69 | 7.86 | 2.2% |
| BI (Abs420) | 0.21 | 0.22 | 4.8% |
| Optimal extraction conditions Organic solvent concentration: 64.9% SMR: 50.6 mL/g SCG d.m. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzezińska, R.; Górska, A.; Wirkowska-Wojdyła, M.; Piasecka, I. Response Surface Methodology as a Tool for Optimization of Extraction Process of Bioactive Compounds from Spent Coffee Grounds. Appl. Sci. 2023, 13, 7634. https://doi.org/10.3390/app13137634
Brzezińska R, Górska A, Wirkowska-Wojdyła M, Piasecka I. Response Surface Methodology as a Tool for Optimization of Extraction Process of Bioactive Compounds from Spent Coffee Grounds. Applied Sciences. 2023; 13(13):7634. https://doi.org/10.3390/app13137634
Chicago/Turabian StyleBrzezińska, Rita, Agata Górska, Magdalena Wirkowska-Wojdyła, and Iga Piasecka. 2023. "Response Surface Methodology as a Tool for Optimization of Extraction Process of Bioactive Compounds from Spent Coffee Grounds" Applied Sciences 13, no. 13: 7634. https://doi.org/10.3390/app13137634
APA StyleBrzezińska, R., Górska, A., Wirkowska-Wojdyła, M., & Piasecka, I. (2023). Response Surface Methodology as a Tool for Optimization of Extraction Process of Bioactive Compounds from Spent Coffee Grounds. Applied Sciences, 13(13), 7634. https://doi.org/10.3390/app13137634