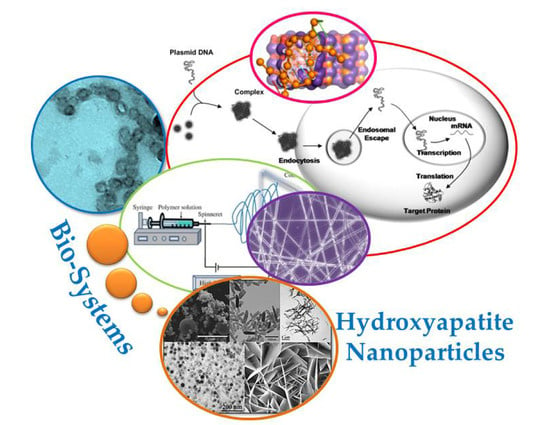
Biodegradable and Biocompatible Systems Based on Hydroxyapatite Nanoparticles
Abstract
:1. Introduction
2. Preparation of HAp Nanoparticles
3. Preparation of Bionanocomposites Incorporating HAp
3.1. Bionanocomposites
3.2. Electrospun Scaffolds Incorporating HAp Nanoparticles
4. Functional HAp Biocomposites through Modification/Encapsulation Techniques
4.1. Antimicrobial Scaffolds Based on HAp
4.2. Modification of HAp Nanoparticles and Doping with Fluorescent and Magnetic Ions for Imaging Applications
4.3. Hydroxyapatite as Non-Viral Gene Delivery System
4.4. Protein Adsorption and Release from HAp Nanoparticles
4.5. Hydroxyapatite Nanoparticles As Carriers for Drug Delivery Systems
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wahl, D.A.; Czernuszka, J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cell. Mater. 2006, 11, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate bioceramics. Ceram. Int. 2015, 41, 13913–13966. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Dorozhkin, S. Calcium orthophosphate-containing bionanocomposites and hybrid materials for biomedical applications. J. Funct. Biomater. 2015, 6, 708–832. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Uskokovic, V.V.; Uskokovic, D.P. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96, 152–191. [Google Scholar] [CrossRef] [PubMed]
- Verron, E.; Khairoun, I.; Guicheux, J.; Bouler, J.M. Calcium phosphate biomaterials as bone drug delivery systems: A review. Drug Discov. Today 2010, 15, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Multiphasic calcium orthophosphate (CaPO4) bioceramics and their biomedical applications. Ceram. Int. 2016, 42, 6529–6554. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal structure of hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Layrolle, P.; Lebugle, A. Characterization and reactivity of nanosized calcium phosphates prepared in anhydrous ethanol. Chem. Mater. 1994, 6, 1996–2004. [Google Scholar] [CrossRef]
- Bakhtiari, L.; Javadpour, J.; Rezaie, H.R.; Erfan, M.; Mazinani, B.; Aminian, A. Pore size control in the synthesis of hydroxyapatite nanoparticles: The effect of pore expander content and the synthesis temperature. Ceram. Int. 2016, 42, 11259–11264. [Google Scholar] [CrossRef]
- Saha, S.K.; Banerjee, A.; Banerjee, S.; Bose, S. Synthesis of nanocrystalline hydroxyapatite using surfactant template systems: Role of templates in controlling morphology. Mater. Sci. Eng. C 2009, 29, 2294–2301. [Google Scholar] [CrossRef]
- Xia, Z.; Liao, L.; Zhao, S. Synthesis of mesoporous hydroxyapatite using a modified hard-templating route. Mater. Res. Bull. 2009, 44, 1626–1629. [Google Scholar] [CrossRef]
- Ye, F.; Guo, H.; Zhang, H.; He, X. Polymeric micelle-templated synthesis of hydroxyapatite hollow nanoparticles for a drug delivery system. Acta Biomater. 2010, 6, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Imura, M.; Nemoto, Y.; Cheng, C.H.; Yamauchi, Y. Block-copolymer-assisted synthesis of hydroxyapatite nanoparticles with high surface area and uniform size. Sci. Technol. Adv. Mater. 2011, 12, 045005. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Chen, K.; Lu, Y. Fabrication and dissolution behavior of hollow hydroxyapatite microspheres intended for controlled drug release. Mater. Res. Bull. 2009, 44, 1939–1942. [Google Scholar] [CrossRef]
- Shum, H.C.; Bandyopadhyay, A.; Bose, S.; Weitz, D.A. Double emulsion droplets as microreactors for synthesis of mesoporous hydroxyapatite. Chem. Mater. 2009, 21, 5548–5555. [Google Scholar] [CrossRef]
- Ma, M.G.; Zhu, J.F. Solvothermal synthesis and characterization of hierarchically nanostructured hydroxyapatite hollow spheres. Eur. J. Inorg. Chem. 2009, 36, 5522–5526. [Google Scholar] [CrossRef]
- Dou, Y.; Cai, S.; Ye, X.; Xu, G.; Hu, H.; Ye, X. Preparation of mesoporous hydroxyapatite films used as biomaterials via sol-gel technology. J. Sol-Gel Sci. Technol. 2012, 61, 126–132. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, L.; Li, Y.; Shi, T. Preparation of irregular mesoporous hydroxyapatite. Mater. Res. Bull. 2008, 43, 1607–1614. [Google Scholar] [CrossRef]
- Ng, S.X.; Guo, J.; Ma, J.; Loo, S.C.J. Synthesis of high surface area mesostructured calcium phosphate particles. Acta Biomater. 2010, 6, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.P.; Yao, Y.; Ning, C.Q.; Guo, Y.J.; Chu, L.F. Fabrication of mesoporous carbonated hydroxyapatite microspheres by hydrothermal method. Mater. Lett. 2011, 65, 2205–2208. [Google Scholar] [CrossRef]
- Guo, Y.P.; Yao, Y.B.; Guo, Y.J.; Ning, C.Q. Hydrothermal fabrication of mesoporous carbonated hydroxyapatite microspheres for a drug delivery system. Microporous Mesoporous Mater. 2012, 155, 245–251. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, J.; Quan, Z.; Yang, P.; Li, C.; Hou, Z.; Lin, J. Hydroxyapatite nano- and microcrystals with multiform morphologies: Controllable synthesis and luminescence properties. Cryst. Growth Des. 2009, 9, 2725–2733. [Google Scholar] [CrossRef]
- Wuthier, R.E.; Rice, G.S.; Wallace, J.E.; Weaver, R.L.; LeGeros, R.Z.; Eanes, E.D. In vitro precipitation of calcium phosphate under intracellular conditions: Formation of brushite from an amorphous precursor in the absence of ATP. Calcif. Tissue Int. 1985, 37, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Leng, Y.; Ding, Y.; Wang, K. Hydrothermal growth of biomimetic carbonated apatite nanoparticles with tunable size, morphology and ultrastructure. Cryst. Eng. Commun. 2013, 15, 2137–2146. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Guo, C.; Qi, Y.; Hu, C. Preparation of ultrahigh-aspect-ratio hydroxyapatite nanofibers in reverse micelles under hydrothermal conditions. Langmuir 2004, 20, 4784–4786. [Google Scholar] [CrossRef] [PubMed]
- López-Macipe, A.; Gómez-Morales, J.; Rodríguez-Clemente, R. Nanosized hydroxyapatite precipitation from homogeneous calcium/citrate/phosphate solutions using microwave and conventional heating. Adv. Mater. 1998, 10, 49–53. [Google Scholar] [CrossRef]
- Ma, M.G. Hierarchically nanostructured hydroxyapatite: Hydrothermal synthesis, morphology control, growth mechanism, and biological activity. Int. J. Nanomed. 2012, 7, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Leena, M.; Rana, D.; Webster, T.J.; Ramalingam, M. Accelerated synthesis of biomimetic nano hydroxyapatite using simulated body fluid. Mater. Chem. Phys. 2016, 180, 166–172. [Google Scholar] [CrossRef]
- McCaffrey, R.; Long, H.; Jin, Y.; Sanders, A.; Park, W.; Zhang, W. Template Synthesis of gold nanoparticles with an organic molecular cage. J. Am. Chem. Soc. 2014, 136, 1782–1785. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Liu, X.Y.; Wang, X.G.; Wang, R.Y. Controlling nanoparticle formation via sizable cages of supramolecular soft materials. Langmuir 2011, 27, 7820–7827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.S.; Liu, X.Y.; Li, J.L.; Xu, H.Y.; Lin, H.; Chen, Y.Y. Design and fabrication of a new class of nano hybrid materials based on reactive polymeric molecular cages. Langmuir 2013, 29, 11498–11505. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chi, W.; Zhao, D.; Liu, H.; Deng, Y. Molecular-cage method: An improvement of the precipitation method in synthesizing nanoparticles. Ind. Eng. Chem. Res. 2016, 55, 8403–8408. [Google Scholar] [CrossRef]
- Nikpour, R.; Rabiee, S.M.; Jahanshahi, M. Synthesis and characterization of hydroxyapatite/chitosan nanocomposite materials for medical engineering applications. Compos. B Eng. 2012, 43, 1881–1886. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Tokuchi, K.; Fukuzaki, H.; Koyama, Y.; Takakuda, K.; Monma, H.; Tanaka, J. Preparation and microstructure analysis of chitosan/hydroxyapatite nanocomposites. J. Biomed. Mater. Res. A. 2000, 55, 20–27. [Google Scholar] [CrossRef]
- Song, X.F.; Ling, F.G.; Chen, X.S. Grafting polymerization of l-lactide on the surface of hydroxyapatite nanoparticles. Acta Polym. Sin. 2013, 1, 95–101. [Google Scholar]
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, N.; Demarchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Aşan-Özüsağlam, M.; Cakmak, Y.S.; Tozak, K.O.; Mol, A.; Menteş, A.; Sezen, G. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotechnol. Bioproc. Eng. 2015, 20, 168–179. [Google Scholar] [CrossRef]
- Kaya, M.; Cakmak, Y.S.; Baran, T.; Asan-Ozusaglam, M.; Mentes, A.; Tozak, K.O. New chitin, chitosan, and O-carboxymethyl chitosan sources from resting eggs of Daphnia longispina (Crustacea); with physicochemical characterization, and antimicrobial and antioxidant activities. Biotechnol. Bioproc. E. 2014, 19, 58–69. [Google Scholar] [CrossRef]
- De Souza Costa-Júnior, E.; Pereira, M.M.; Mansur, H.S. Properties and biocompatibility of chitosan films modified by blending with PVA and chemically crosslinked. J. Mater. Sci. Mater. Med. 2009, 20, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Zhao, Y.; Shen, Q.; Wang, D.J.; Xu, D.F. The effect of carboxymethyl-chitosan on the precipitation of calcium carbonate. J. Cryst. Growth 2004, 261, 571–576. [Google Scholar] [CrossRef]
- Dumont, V.C.; Mansur, A.A.P.; Carvalho, S.M.; Medeiros Borsagli, F.G.L.; Pereira, M.M.; Mansur, H.S. Chitosan and carboxymethyl-chitosan capping ligands: Effects on the nucleation and growth of hydroxyapatite nanoparticles for producing biocomposite membranes. Mater. Sci. Eng. C 2016, 59, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Barna, A.S.; Ciobanu, G.; Luca, C.; Luca, A.C. Nanohydroxyapatite—Calcium fructoborate composites: Synthesis and characterization. Rev. Chim. 2015, 66, 1618–1621. [Google Scholar]
- Janicki, P.; Schmidmaier, G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury 2011, 42, S77–S81. [Google Scholar] [CrossRef] [PubMed]
- Zadegan, S.; Hosainalipour, M.; Rezaie, H.R.; Ghassai, H.; Shokrgozar, M.A. Synthesis and biocompatibility evaluation of cellulose/hydroxyapatite nanocomposite scaffold in 1-n-allyl-3-methyl imidazolium chloride. Mater. Sci. Eng. C 2011, 31, 954–961. [Google Scholar] [CrossRef]
- Zimmermann, K.A.; LeBlanc, J.M.; Sheets, K.T.; Fox, R.W.; Gatenholm, P. Biomimetic design of a bacterial cellulose/hydroxyapatite nanocomposite for bone healing applications. Mater. Sci. Eng. C 2011, 31, 43–49. [Google Scholar] [CrossRef]
- He, M.; Chang, C.; Peng, N.; Zhang, L. Structure and properties of hydroxyapatite/cellulose nanocomposite films. Carbohyd. Polym. 2012, 87, 2512–2518. [Google Scholar] [CrossRef]
- Duarte, E.B.; das Chagas, B.S.; Andrade, F.A.; Brígida, A.I.S.; Borges, M.F.; Muniz, C.R.; Souza Filho, M.S.M.; Morais, J.P.S.; Feitosa, J.P.A.; Rosa, M.F. Production of hydroxyapatite–bacterial cellulose nanocomposites from agroindustrial wastes. Cellulose 2015, 22, 3177–3187. [Google Scholar] [CrossRef]
- Wu, J.M.; Liu, R.H. Cost-effective production of bacterial cellulose in static cultures using distillery wastewater. J. Biosci. Bioeng. 2013, 115, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Lin, D.Y.; Johnson, M.A.; Vohden, R.A.; Chen, D.; Martin, D.C. Tailored nanofiber morphologies using modulated electrospinning for biomedical applications. Mater. Res. Soc. Symp. Proc. 2003, 736, D3.8.1–D3.8.6. [Google Scholar] [CrossRef]
- Crespy, D.; Friedemann, K.; Popa, A.M. Colloid-electrospinning: Fabrication of multicompartment nanofibers by the electrospinning of organic or/and inorganic dispersions and emulsions. Macromol. Rapid Commun. 2012, 33, 1978–1995. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Greiner, A. On the way to clean and safe electrospinning-green electrospinning: Emulsion and suspension electrospinning. Polym. Adv. Technol. 2011, 22, 372–378. [Google Scholar] [CrossRef]
- Pal, J.; Sharma, S.; Sanwaria, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Conducive 3D porous mesh of poly(ε-caprolactone) made via emulsion electrospinning. Polymer 2014, 55, 3970–3979. [Google Scholar] [CrossRef]
- Yang, F.; Both, S.; Yang, X.; Walboomers, X.; Jansen, J. Development of an electrospun nano-apatite/PCL composite membrane for GTR/GBR application. Acta Biomater. 2009, 5, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Ling, F.; Ma, L.; Yang, C.; Chen, X. Electrospun hydroxyapatite grafted poly(l-lactide)/poly(lactic-co-glycolic acid) nanofibers for guided bone regeneration membrane. Compos. Sci. Technol. 2013, 79, 8–14. [Google Scholar] [CrossRef]
- Yang, T.; Cui, X.; Kao, Y.; Wang, H.; Wen, J. Electrospinning PTMC/Gt/OA-HA composite fiber scaffolds and the biocompatibility with mandibular condylar chondrocytes. Colloids Surfaces A 2016, 499, 123–130. [Google Scholar] [CrossRef]
- Jeong, S.I.; Ko, E.K.; Yum, J.; Jung, C.H.; Lee, Y.M.; Shin, H. Nanofibrous poly(lactic acid)/hydroxyapatite composite scaffolds for guided tissue regeneration. Macromol. Biosci. 2008, 8, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Novotna, K.; Zajdlova, M.; Suchy, T.; Hadraba, D.; Lopot, F.; Zaloudkova, M.; Douglas, T.E.L.; Munzarova, M.; Juklickova, M.; Stranska, D.; et al. Polylactide nanofibers with hydroxyapatite as growth substrates for osteoblast-like cells. J. Biomed. Mater. Res. A 2014, 102, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Bombonato-Prado, K.F.; Bellesini, L.S.; Junta, C.M.; Marques, M.M.; Passos, G.A.; Rosa, A.L. Microarray-based gene expression analysis of human osteoblasts in response to different biomaterials. J. Biomed. Mater. Res. A 2009, 88, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.M.; McCarthy, G.M.; Sallis, J.D.; Morgan, M.P. Phosphocitrate inhibits calcium hydroxyapatite induced mitogenesis and upregulation of matrix metalloproteinase-1, interleukin-1beta and cyclooxygenase-2 mRNA in human breast cancer cell lines. Breast Cancer Res. Treat. 2003, 79, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Markel, D.C.; Wang, S.; Shi, T.; Mao, G.; Ren, W. Electrospun polyvinyl alcohol-collagen-hydroxyapatite nanofibers: A biomimetic extracellular matrix for osteoblastic cells. Nanotechnology 2012, 23, 115101. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Liu, Y.T.; Tang, S.; Sun, J.L.; Guo, Y.P.; Zhu, Z.A. Hydrothermal fabrication of hydroxyapatite/chitosan/carbon porous scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B 2014, 102, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Leszczak, V.; Place, L.W.; Franz, N.; Popat, K.C.; Kipper, M. Nanostructured Biomaterials from Electrospun Demineralized Bone-Matrix: A Survey of Processing and Crosslinking Strategies. J. Appl. Mater. Interfaces 2014, 6, 9328–9337. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.H.; El-Fiqi, A.; Kim, J.H.; Yun, Y.R.; Jang, J.H.; Han, C.M.; Lee, E.J.; Kim, H.W. Biointerface control of electrospun fiber scaffolds for bone regeneration: Engineered protein link to mineralized surface. Acta Biomater. 2014, 10, 2750–2761. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Jin, X.; Ma, P.X. Calcium phosphate deposition rate, structure and osteoconductivity on electrospun poly(l-lactic acid) matrix using electrodeposition or simulated body fluid incubation. Acta Biomater. 2014, 10, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Sun, G. Prevention of hospital and community acquired infections by using antibacterial textiles and clothing. In Polymeric Materials with Antimicrobial Activity; Muñoz-Bonilla, A., Cerrada, M.L., Fernández-García, M., Eds.; RSC Polymer Chemistry Series: London, UK, 2013; Chapter 6; pp. 139–155. [Google Scholar] [CrossRef]
- Lenz, A.M.; Fairweather, M.; Cheadle, W.G. Resistance profiles in surgical-site infection. Future Microbiol. 2008, 3, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Broex, E.C.; van Asselt, A.D.; Bruggeman, C.A.; van Tiel, F.H. Surgical site infections: How high are the costs? J. Hosp. Infect. 2009, 72, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Stamm, W.E. Infections related to medical devices. Ann. Intern. Med. 1978, 89, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Control Release 2008, 130, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Schnieders, J.; Gbureck, U.; Thull, R.; Kissel, T. Controlled release of gentamicin from calcium phosphate-poly(lactic acid-co-glycolic acid) composite bone cement. Biomaterials 2006, 27, 4239–4249. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.B.; Bard, A.J. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, W.J.; Rosenberg, H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J. Bacteriol. 1982, 152, 7–13. [Google Scholar] [PubMed]
- Yang, W.; Shen, C.; Ji, Q.; An, H.; Wang, J.; Liu, Q.; Zhang, Z. Food storage material silver nanoparticles interfere with DNA replication fidelity and bind with DNA. Nanotechnology 2009, 20, 085102. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.; Kim, J.; Park, S.; Lee, H.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Rameshbabu, N.; Sampath Kumar, T.S.; Prabhakar, T.G.; Sastry, V.S.; Murty, K.V.G.K.; Prasad Rao, K. Antibacterial nanosized silver substituted hydroxyapatite: Synthesis and characterization. J. Biomed. Mater. Res. A 2007, 80, 581–591. [Google Scholar] [CrossRef]
- Kim, T.N.; Feng, Q.L.; Kim, J.O.; Wu, J.; Wang, H.; Chen, G.C.; Cui, F.Z. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J. Mater. Sci. Mater. Med. 1998, 9, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.N.; Chang, L.; Thian, E.S. Development of nanosized silver-substituted apatite for biomedical applications: A review. Nanomed. NBM 2015, 11, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Rauschmann, M.A.; Wichelhaus, T.A.; Stirnal, V.; Dingeldein, E.; Zichner, L.; Schnettler, R.; Alt, V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials 2005, 26, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.C.; Zhu, Y.J.; Chen, F.F.; Sun, T.W.; Shen, Y.Q. One-step synthesis of silver nanoparticle-decorated hydroxyapatite nanowires for the construction of highly flexible free-standing paper with high antibacterial activity. Chemistry 2016, 22, 11224–11231. [Google Scholar] [CrossRef] [PubMed]
- Livitska, O.; Struynska, N.; Zatovsky, I.; Nikolenko, I.; Slobodyanik, N.; Prylutskyy, Y.; Epple, M.; Prymak, O.; Byeda, A. Copper(II), zinc(II) and copper(II)/zinc(II)-containing carbonate-substituted hydroxyapatite: Synthesis, characterization and thermal behaviour. Mater. Werkstofftech. 2016, 47, 85–91. [Google Scholar] [CrossRef]
- Shannmugam, S.; Gopal, B. Copper substituted hydroxyapatite and fluorapatite; Synthesis, characterization and antimicrobial properties. Ceram. Int. 2014, 40, 15655–15662. [Google Scholar] [CrossRef]
- Niu, N.; Wang, D.; Huang, S.; Li, C.; He, F.; Gai, S.; Li, X.; Yang, P. Controlled synthesis of luminescent F-substituted strontium hydroxyapatite with hierarchical structures for drug delivery. Cryst. Eng. Commun. 2012, 14, 1744–1752. [Google Scholar] [CrossRef]
- Xu, Z.; Li, C.; Hou, Z.; Yang, D.; Kang, X.; Lin, J. Facile synthesis of an up-conversion luminescent and mesoporous Gd2O3: Er3+@nSiO2@mSiO2 nanocomposite as a drug carrier. Nanoscale 2011, 3, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, L.L.; Zhao, W.H.; Chen, Y.X.; Wang, X.; Fang, C.J.; Feng, W.; Zhang, T.L.; Lu, M.; Peng, S.; et al. The intracellular controlled release from bioresponsive mesoporous silica with folate as both targeting and capping agent. Nanoscale 2012, 4, 3577–3583. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Heng, C.; Zheng, X.; Liu, M.; Xu, D.; Huang, H.; Deng, F.; Hui, J.; Zhang, X.; Wei, Y. Fabrication of luminescent hydroxyapatite nanorods through surface-initiated RAFT polymerization: Characterization, biological imaging and drug delivery applications. Appl. Surface Sci. 2016, 386, 269–275. [Google Scholar] [CrossRef]
- Hui, J.; Wang, X. Hydroxyapatite nanocrystals: Colloidal chemistry, assembly and their biological applications. Inorg. Chem. Front. 2014, 1, 215–225. [Google Scholar] [CrossRef]
- Hui, J.; Zhang, X.; Zhang, Z.; Wang, S.; Tao, L.; Wei, Y.; Wang, X. Fluoridated HAp:Ln3+ (Ln = Eu or Tb) nanoparticles for cell-imaging. Nanoscale 2012, 4, 6967–6970. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, M.; Hui, J.; Fan, D.; Ma, H.; Zhang, X.; Wang, Y.; Wei, Y. Ln3+-doped hydroxyapatite nanocrystals: Controllable synthesis and cell imaging. Phys. Chem. Chem. Phys. 2015, 17, 20301–20307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hui, J.; Yang, B.; Yang, Y.; Fan, D.; Liu, M.; Tao, L.; Wei, Y. PEGylation of fluoridated hydroxyapatite (FAp): Ln3+ nanorods for cell imaging. Polym. Chem. 2013, 4, 4120–4125. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Rufaihah, A.J.; Zhang, Y. Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials 2008, 29, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, K.; Liu, M.; Zhang, X.; Tao, L.; Chen, Y.; Wei, Y. Polymeric AIE-based nanoprobes for biomedical applications: Recent advances and perspectives. Nanoscale 2015, 7, 11486–11508. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liang, Z.; Chen, J.; Yu, J.; Xu, R. AIE luminogen bridged hollow hydroxyapatite nanocapsules for drug delivery. Dalton Trans. 2013, 42, 9877–9883. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.A.; Singh, A.K.; Sharma, P.; Brown, S.C.; Moudgil, B.M. Nanoparticles as contrast agents for in vivo bioimaging: Current status and future perspectives. Anal. Bioanal. Chem. 2011, 399, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Fukumori, Y.; Ichikawa, H. Nanoparticles for cancer therapy and diagnosis. Adv. Powder Technol. 2006, 17, 1–28. [Google Scholar] [CrossRef]
- Laranjeira, M.S.; Moço, A.; Ferreira, J.; Coimbra, S.; Costa, E.; Santos-Silva, A.; Ferreira, P.J.; Monteiro, F.J. Different hydroxyapatite magnetic nanoparticles for medical imaging: Its effects on hemostatic, hemolytic activity and cellular cytotoxicity. Colloid Surface B 2016, 146, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Panseri, S.; Cunha, C.; D’Alessandro, T.; Sandri, M.; Russo, A.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Magnetic hydroxyapatite bone substitutes to enhance tissue regeneration: Evaluation in vitro using osteoblast-like cells and in vivo in a bone defect. PLoS ONE 2012, 7, e38710. [Google Scholar] [CrossRef] [PubMed]
- Inukai, A.; Sakamoto, N.; Aono, H.; Sakurai, O.; Shinozaki, K.; Suzuki, H.; Wakiya, N. Synthesis and hyperthermia property of hydroxyapatite–ferrite hybrid particles by ultrasonic spray pyrolysis. J. Magn. Magn. Mater. 2011, 323, 965–969. [Google Scholar] [CrossRef]
- Aval, N.A.; Islamian, J.P.; Hatamian, M.; Arabfirouzjaei, M.; Javadpour, J.; Rashidi, M.R. Doxorubicin loaded large-pore mesoporous hydroxyapatite coated superparamagnetic Fe3O4 nanoparticles for cancer treatment. Int. J. Pharm. 2016, 509, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Roy, A.; Hong, D.; Kumta, P.N. Nanostructured silicate substituted calcium phosphate (NanoSiCaPs) nanoparticles—Efficient calcium phosphate based non-viral gene delivery systems. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Truong-Le, V.L.; Walsh, S.M.; Schwabert, E.; Mao, H.Q.; Guggino, W.B.; August, J.T.; Leong, K.W. Gene transfer by DNA-gelatin nanospheres. Arch. Biochem. Biophys. 1999, 361, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Orrantia, E.; Chang, P.L. Intracellular-distribution of DNA internalized through calcium-phosphate precipitation. Exp. Cell Res. 1990, 190, 170–174. [Google Scholar] [CrossRef]
- James, R.F.L.; Grosveld, F.G. DNA-mediated gene transfer into mammalian cells. In Techniques in Molecular Biology; Walker, J.M., Gaastra, W., Eds.; Croom Helm: London, UK, 1987; Volume 2, pp. 187–202. [Google Scholar]
- Roy, I.; Mitra, S.; Maitra, A.; Mozumdar, S. Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery. Int. J. Pharm. 2003, 250, 25–33. [Google Scholar] [CrossRef]
- Bisht, S.; Bhakta, G.; Mitra, S.; Maitra, A. pDNA loaded calcium phosphate nanoparticles: Highly efficient non-viral vector for gene delivery. Int. J. Pharm. 2005, 288, 157–168. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Saltzmann, W.M. Synthetic DNA delivery systems. Nat. Biotechnol. 2000, 18, 33–37. [Google Scholar] [PubMed]
- Lee, K.; Oh, M.H.; Lee, M.S.; Nam, Y.S.; Park, T.G.; Jeong, J.H. Stabilized calcium phosphate nano-aggregates using a dopa-chitosan conjugate for gene delivery. Int. J. Pharm. 2013, 445, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.H.; Kunou, M.; Nagaoka, M.; Kundu, A.K.; Hoshiba, T.; Akaike, T. High-efficiency gene delivery for expression in mammalian cells by nanoprecipitates of Ca–Mg phosphate. Gene 2004, 341, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.H. Fluoride enhances transfection activity of carbonate apatite by increasing cytoplasmic stability of plasmid DNA. Biochem. Biophys. Res. Commun. 2011, 409, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Hanifi, A.; Fathi, M.H.; Sadeghi, H.M.M. Effect of strontium ions substitution on gene delivery related properties of calcium phosphate nanoparticles. J. Mater. Sci. Mater. Med. 2010, 21, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A.; Revell, P.A.; Smith, N.; Buckland, T. Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 2006, 27, 5014–5026. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.E.; Patel, N.; Skepper, J.N.; Best, S.M.; Bonfield, S.M.W. Comparison of in vivo dissolution processes in hydroxyapatite and silicon-substituted hydroxyapatite bioceramics. Biomaterials 2003, 24, 4609–4620. [Google Scholar] [CrossRef]
- Marchat, D.; Zymelka, M.; Coelho, C.; Gremillard, L.; Joly-Pottuz, L.; Babonneau, F.; Esnouf, C.; Chevalier, J.; Bernache-Assollant, D. Accurate characterization of pure silicon-substituted hydroxyapatite powders synthesized by a new precipitation route. Acta Biomater. 2013, 9, 6992–7004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolova, V.; Prymak, O.; Meyer-Zaika, W.; Cölfen, H.; Rehage, H.; Shukla, A.; Epple, M. Synthesis and characterisation of DNA-functionalised calcium phosphate nanoparticles. Mater. Werkstofftech. 2006, 37, 441–445. [Google Scholar] [CrossRef]
- Tenkumo, T.; Rotan, O.; Sokolova, V.; Epple, M. Protamine increases transfection efficiency and cell viability after transfection with calcium phosphate nanoparticles. Nano Biomed. 2013, 5, 64–74. [Google Scholar]
- Tenkumo, T.; Vanegas Sáenz, J.R.; Takada, Y.; Takahashi, M.; Rotan, O.; Sokolova, V.; Epple, M.; Sasaki, K. Gene transfection of human mesenchymal stem cells with a nano-hydroxyapatite–collagen scaffold containing DNA-functionalized calcium phosphate nanoparticles. Genes Cells 2016, 21, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Revilla-López, G.; Casanovas, J.; Bertran, O.; Turon, P.; Puiggalí, J.; Alemán, C. Modeling biominerals formed by apatites and DNA. Biointerphases 2013, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turon, P.; del Valle, L.J.; Alemán, C.; Puiggalí, J. Preparation and applications of hydroxyapatite nanocomposites based on biodebradable and natural polymers. In Synthesis Techniques for Polymer Nanocomposites; Mittal, V., Ed.; Wiley-VCH: Weinheim, Germany, 2015; pp. 51–85. [Google Scholar]
- Bertran, O.; del Valle, L.J.; Revilla-López, G.; Chaves, G.; Cardús, L.; Casas, M.T.; Casanovas, J.; Turon, P.; Puiggalí, J.; Alemán, C. Mineralization of DNA into nanoparticles of hydroxyapatite. Dalton Trans. 2014, 43, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Valle, L.J.; Bertran, O.; Chaves, G.; Revilla-López, G.; Rivas, M.; Casas, M.T.; Casanovas, J.; Turon, P.; Puiggalí, J.; Alemán, C. DNA adsorbed on hydroxyapatite surfaces. J. Mater. Chem. B 2014, 2, 6953–6966. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, V. Dissolution mechanism of calcium apatites in acids: A review of literature. World J. Methodol. 2012, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bertran, O.; Revilla-López, G.; Casanovas, J.; del Valle, L.J.; Turon, P.; Puiggalí, J.; Alemán, C. Dissolving hydroxyolite: A DNA molecule into its hydroxyapatite mold. Chem. Eur. J. 2016, 22, 6631–6636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turon, P.; Puiggalí, J.; Bertran, O.; Alemán, C. Surviving mass extinctions through biomineralized DNA. Chem. Eur J. 2015, 21, 18892–18898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.K.; Cao, B.R.; Mao, C.B. Bacteriophage bundles with prealigned Ca2+ initiate the oriented nucleation and growth of hydroxylapatite. Chem. Mater. 2010, 22, 3630–3636. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Abbineni, G.; Cao, B.R.; Mao, C.B. Nanofibrous bio-inorganic hybrid structures formed through self-assembly and oriented mineralization of genetically engineered phage nanofibers. Small 2010, 6, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Yang, M.; Mao, C. Phage as a genetically modifiable supramacromolecule in chemistry, materials and medicine. Acc. Chem. Res. 2016, 49, 1111–1120. [Google Scholar] [CrossRef]
- Xu, H.; Cao, B.R.; George, A.; Mao, C.B. Self-assembly and mineralization of genetically modifiable biological nanofibers driven by β-structure formation. Biomacromolecules 2011, 12, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Chao, H.S.; Liu, H.L.; Liu, H.S. Stability of hen egg white lysozyme during denaturation is enhanced by pretreatment with supercritical carbon dioxide. J. Biosci. Bioeng. 2009, 107, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Arulandu, A.; Struck, D.K.; Swanson, S.; Sacchettini, J.C.; Young, R. Disulfide isomerization after membrane release of its SAR domain activates P1 lysozyme. Science 2005, 307, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Bakowsky, U.; Rytting, E.; Schaper, A.K.; Kissel, T. Charged nanoparticles as protein delivery systems: A feasibility study using lysozyme as model protein. Eur. J. Pharm. Biopharm. 2008, 69, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Nagadome, H.; Kawano, K.; Terada, Y. Identification of the adsorbing site of lysozyme onto the hydroxyapatite surface using hydrogen exchange and 1H-NMR. FEBS Lett. 1993, 317, 128–130. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, H.; Zhang, S. Lysozyme loading and release from Se doped hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2016, 61, 545–552. [Google Scholar] [CrossRef]
- Ishihara, S.; Matsumoto, T.; Onoki, T.; Uddin, M.H.; Sohmura, T.; Nakahira, A. Regulation of the protein-loading capacity of hydroxyapatite by mercaptosuccinic acid modification. Acta Biomater. 2010, 6, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Loo, C.Y.; Van, K.L.; Zavgorodniy, A.V.; Rohanizadeh, R. Modulating protein adsorption onto hydroxyapatite particles using different amino acid treatments. J. R. Soc. Interface 2012, 9, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Loo, C.Y.; Zavgorodniy, A.V.; Ghadiri, M.; Rohanizadeh, R. A novel approach to enhance protein adsorption and cell proliferation on hydroxyapatite: Citric acid treatment. RSC Adv. 2013, 3, 4040–4051. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Guo, S.; Shi, J.; Du, W.; Wang, Z.; Ye, L.; Gu, W. Biomimetic mineralization of nano-sized, needle-like hydroxyapatite with ultrahigh capacity for lysozyme adsorption. Mater. Sci. Eng. C 2016, 68, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Shu, S.H.; Shih, Y.J.; Chu, C.W.; Ruaan, R.C.; Chen, W.Y. Hemocompatible mixed-charge copolymer brushes of pseudozwitterionic surfaces resistant to nonspecific plasma protein fouling. Langmuir 2010, 26, 3522–3530. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Hamada, Y.; Taira, M.; Takahashi, J. Crystallinity and solubility characteristics of hydroxyapatite adsorbed amino acid. Biomaterials 2002, 23, 2241–2247. [Google Scholar] [CrossRef]
- Heiss, A.; DuChesne, A.; Denecke, B.; Grötzinger, J.; Yamamoto, J.K.; Renné, T.; Jahnen-Dechent, W. A structural basis of calcification inhibition by alpha(2)-HS glycoprotein/fetuin-A—Formation of colloidal calciprotein particles. J. Biol. Chem. 2003, 278, 13333–13341. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.C.; Harshita, Mishra P.K.; Talegaonkar, S. Ceramic nanoparticles: Fabrication methods and applications in drug delivery. Curr. Pharm. Des. 2015, 21, 6165–6188. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.C.; Sharma, H.; Rawat, P.; Verma, A.; Leekha, A.; Kumar, V.; Tyagi, A.; Gurjar, B.S.; Iqbal, Z.; Talegaonkar, S. Synergistic anticancer efficacy of bendamustine hydrochloride loaded bioactive hydroxyapatite nanoparticles: In-vitro, ex-vivo and in-vivo evaluation. Colloids Surf. B Biointerface 2016, 146, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Tanaka, Y.; Czernuszka, J.T. Encapsulation and release of a hydrophobic drug from hydroxyapatite coated liposomes. Biomaterials 2007, 28, 2687–2694. [Google Scholar] [CrossRef] [PubMed]
- Leu, C.T.; Luegmayr, E.; Freedman, L.P.; Rodan, G.A.; Reszka, A.A. Relative binding affinities of bisphosphonates for human bone and relationship to antiresorptive efficacy. Bone 2006, 38, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Lee, J.S.; Ryu, T.K.; Kang, R.H.; Jeong, K.Y.; Jun, D.R.; Koh, J.M.; Kim, S.E.; Choi, S.W. Alendronate-modified hydroxyapatite nanoparticles for bone-specific dual delivery of drug and bone mineral. Macromol. Res. 2016, 24, 623–628. [Google Scholar] [CrossRef]
- Liang, Y.H.; Liu, C.H.; Liao, S.H.; Lin, Y.Y.; Tang, H.W.; Liu, S.Y.; Lai, I.R.; Wu, K.C. Cosynthesis of cargo-loaded hydroxyapatite/alginate core–shell nanoparticles (HAP@Alg) as pH-responsive nanovehicles by a pre-gel method. ACS Appl. Mater. Interfaces 2012, 4, 6720–6727. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yuan, Y.; Liu, C.; Wu, Y.; Lu, X.; Qian, J. Differential cytotoxicity and particle action of hydroxyapatite nanoparticles in human cancer cells. Nanomedicine 2014, 9, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, S.; Cao, X.; Yuan, L.; Wang, Y.; Yin, Y.; Qiu, T.; Dai, H.; Wang, X. Different inhibitory effect and mechanism of hydroxyapatite nanoparticles on normal cells and cancer cells in vitro and in vivo. Sci. Rep. 2014, 4, 7134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.S.; Tang, S.L.; Ai, Z.L. Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human hepatoma BEL-7402 cells. World J. Gastroenterol. 2003, 9, 1968–1971. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, B.; Fan, H.S.; Zhang, X.D. Preparation of nano-hydroxyapatite particles with different morphology and their response to highly malignant melanoma cells in vitro. Appl. Surf. Sci. 2008, 255, 357–360. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, C.; Qian, J.; Wang, J.; Zhang, Y. Size-mediated cytotoxicity and apoptosis of hydroxyapatite nanoparticles in human hepatoma HepG2 cells. Biomaterials 2010, 31, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, S.H.; Zhang, Q.H.; Liu, F.Y.; Wu, P. 23,24-Dihydrocucurbitacin B induces G2/M cell-cycle arrest and mitochondria-dependent apoptosis in human breast cancer cells (Bcap37). Cancer Lett. 2007, 256, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liang, T.; Liu, C.; Yuan, Y.; Qian, J. Correlation of particle properties with cytotoxicity and cellular uptake of hydroxyapatite nanoparticles in human gastric cancer cells. Mater. Sci. Eng. C 2016, 67, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P.; Puvvada, N.; Dash, R.; Prashanth Kumar, B.N.; Sarkar, D.; Azab, B.; Pathak, A.; Kundu, S.C.; Fisher, P.B.; et al. The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials 2011, 32, 3794–3806. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Yang, H.C.; Hon, M.H. The effect of the molecular weight of chitosan nanoparticles and its application on drug delivery. Microchem. J. 2009, 92, 87–91. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Wen, X.; Zhou, S.; Tong, X.; Su, P.; Li, H.; Shi, D. Anti-tumor activity of paclitaxel-loaded chitosan nanoparticles: An in vitro study. Mater. Sci. Eng. C 2009, 29, 2392–2397. [Google Scholar] [CrossRef]
- Victor, S.P.; Paul, W.; Vineeth, V.M.; Komeri, R.; Jayabalan, M.; Sharma, C.P. Neodymium doped hydroxyapatite theranostic nanoplatforms for colon specific drug delivery applications. Colloid. Surf. B Biointerfaces 2016, 145, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Zöller, M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Du, S.; Ni, J.; Zhou, J.; Yao, J. Mitochondria and nuclei dual-targeted heterogeneous hydroxyapatite nanoparticles for enhancing therapeutic efficacy of doxorubicin. Biomaterials 2016, 94, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Dell’Arciprete, M.L.; Wegmann, M.; Distel, L.V.; Neuhuber, W.; Gonzalez, M.C.; Kryschi, C. Oxidized silicon nanoparticles for radiosensitization of cancer and tissue cells. Biochem. Biophys. Res. Commun. 2013, 434, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles—A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Hanagata, N.; Ikoma, T.; Huang, J.Y.; Li, K.Y.; Lin, C.P.; Lin, F.H. Hafnium-doped hydroxyapatite nanoparticles with ionizing radiation for lung cancer treatment. Acta Biomater. 2016, 37, 165–173. [Google Scholar] [CrossRef] [PubMed]















© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turon, P.; Del Valle, L.J.; Alemán, C.; Puiggalí, J. Biodegradable and Biocompatible Systems Based on Hydroxyapatite Nanoparticles. Appl. Sci. 2017, 7, 60. https://doi.org/10.3390/app7010060
Turon P, Del Valle LJ, Alemán C, Puiggalí J. Biodegradable and Biocompatible Systems Based on Hydroxyapatite Nanoparticles. Applied Sciences. 2017; 7(1):60. https://doi.org/10.3390/app7010060
Chicago/Turabian StyleTuron, Pau, Luís J. Del Valle, Carlos Alemán, and Jordi Puiggalí. 2017. "Biodegradable and Biocompatible Systems Based on Hydroxyapatite Nanoparticles" Applied Sciences 7, no. 1: 60. https://doi.org/10.3390/app7010060
APA StyleTuron, P., Del Valle, L. J., Alemán, C., & Puiggalí, J. (2017). Biodegradable and Biocompatible Systems Based on Hydroxyapatite Nanoparticles. Applied Sciences, 7(1), 60. https://doi.org/10.3390/app7010060