Effects of Low Intensity Continuous Ultrasound (LICU) on Mouse Pancreatic Tumor Explants
Abstract
:1. Introduction
2. Materials and Methods
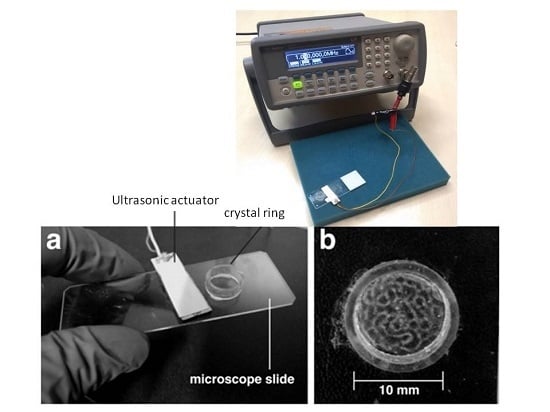
2.1. Ultrasound Exposure System: Chamber of Acoustic Actuation
2.2. Pressure Amplitude Measurements
2.3. Attenuation of the Ultrasounds on the Samples
2.4. Thermal Measurements within the Acoustic Chamber
2.5. Tumor Explant Preparation
2.6. Immunofluorescence Staining of PANC-1 Tumor Explants
2.7. ELISAs for Inflammation
2.8. Image Acquisition
2.9. Statistical Analysis
3. Results and Discussion
3.1. Mechanical and Thermal Effects on the Samples
3.2. LICU Effects on Cytokine Secretion, Tumor Vasculature, and Collagen I Production
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kristiansen, T.K.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Roe, L.R. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound: A multicenter, prospective, randomized, double-blind, placebo-controlled study. J. Bone Jt. Surg. Am. 1997, 79, 961–973. [Google Scholar] [CrossRef]
- Busse, J.W.; Bhandari, M.; Kulkarni, A.V.; Tunks, E. The effect of low-intensity pulsed ultrasound therapy on time to fracture healing: A meta-analysis. Can. Med. Assoc. J. 2002, 166, 437–441. [Google Scholar]
- Claes, L.; Willie, B. The enhancement of bone regeneration by ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Mundi, R.; Petis, S.; Kaloty, R.; Shetty, V.; Bhandari, M. Low-intensity pulsed ultrasound: Fracture healing. Indian J. Orthop. 2009, 43, 132–140. [Google Scholar] [PubMed]
- Xin, Z.; Lin, G.; Lei, H.; Lue, T.F.; Guo, Y. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. Transl. Androl. Urol. 2016, 5, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Greenbaum, L.D.; Goldberg, B.B. Contrast-Enhanced Ultrasound:What Is the Evidence and What Are the Obstacles? Am. J. Roentgenol. 2009, 193, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Helfield, B.; Chen, X.; Watkins, S.C.; Villanueva, F.S. Biophysical insight into mechanisms of sonoporation. Proc. Natl. Acad. Sci. USA 2016, 113, 9983–9988. [Google Scholar] [CrossRef] [PubMed]
- El-Bialy, T.; Alhadlaq, A.; Wong, B.; Kucharski, C. Ultrasound effect on neural differentiation of gingival stem/progenitor cells. Ann. Biomed. Eng. 2014, 42, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, R.; Masaki, C.; Toshinaga, A.; Okinaga, T.; Nishihara, T.; Yamanaka, N.; Nakamoto, T.; Hosokawa, R. The effects of low-intensity pulsed ultrasound exposure on gingival cells. J. Periodontol. 2011, 82, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Harle, J.; Salih, V.; Mayia, F.; Knowles, J.C.; Olsen, I. Effects of ultrasound on the growth and function of bone and periodontal ligament cells in vitro. Ultrasound Med. Biol. 2001, 27, 579–586. [Google Scholar] [CrossRef]
- Ren, L.; Yang, Z.; Song, J.; Wang, Z.; Deng, F.; Li, W. Involvement of p38 mapk pathway in low intensity pulsed ultrasound induced osteogenic differentiation of human periodontal ligament cells. Ultrasonics 2013, 53, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, Y.; Zhou, J.; Li, J.; Deng, F.; Wang, Z.; Song, J. Low-intensity pulsed ultrasound stimulation facilitates osteogenic differentiation of human periodontal ligament cells. PLoS ONE 2014, 9, e95168. [Google Scholar] [CrossRef] [PubMed]
- Dalla-Bona, D.A.; Tanaka, E.; Oka, H.; Yamano, E.; Kawai, N.; Miyauchi, M.; Takata, T.; Tanne, K. Effects of ultrasound on cementoblast metabolism in vitro. Ultrasound Med. Biol. 2006, 32, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Dalla-Bona, D.A.; Tanaka, E.; Inubushi, T.; Oka, H.; Ohta, A.; Okada, H.; Miyauchi, M.; Takata, T.; Tanne, K. Cementoblast response to low- and high-intensity ultrasound. Arch. Oral. Biol. 2008, 53, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Ito, H.; Nakagawa, Y.; Akiyama, H.; Miyamoto, M.; Nakamura, T. Transforming growth factor-beta1 mediates the effects of low-intensity pulsed ultrasound in chondrocytes. Ultrasound Med. Biol. 2005, 31, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, R.; Ryo, A.; Komitsu, N.; Mikuni-Takagaki, Y.; Fukui, A.; Takagi, Y.; Shiraishi, T.; Morishita, S.; Yamazaki, Y.; Kumagai, K.; et al. Low-intensity pulsed ultrasound activates the phosphatidylinositol 3 kinase/akt pathway and stimulates the growth of chondrocytes in three-dimensional cultures: A basic science study. Arthritis Res. Ther. 2008, 10, R77. [Google Scholar] [CrossRef] [PubMed]
- Schumann, D.; Kujat, R.; Zellner, J.; Angele, M.K.; Nerlich, M.; Mayr, E.; Angele, P. Treatment of human mesenchymal stem cells with pulsed low intensity ultrasound enhances the chondrogenic phenotype in vitro. Biorheology 2006, 43, 431–443. [Google Scholar] [PubMed]
- Angle, S.R.; Sena, K.; Sumner, D.R.; Virdi, A.S. Osteogenic differentiation of rat bone marrow stromal cells by various intensities of low-intensity pulsed ultrasound. Ultrasonics 2011, 51, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Nakao, J.; Fujii, Y.; Kusuyama, J.; Bandow, K.; Kakimoto, K.; Ohnishi, T.; Matsuguchi, T. Low-intensity pulsed ultrasound (LIPUS) inhibits LPS-induced inflammatory responses of osteoblasts through TLR4-MyD88 dissociation. Bone 2014, 58, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Kim, J.; Seonwoo, H.; Park, S.H.; Choung, P.-H.; Chung, J.H. In vitro effects of low-intensity pulsed ultrasound stimulation on the osteogenic differentiation of human alveolar bone-derived mesenchymal stem cells for tooth tissue engineering. Biomed. Res. Int. 2013, 2013, 269724. [Google Scholar] [PubMed]
- Al-Daghreer, S.; Doschak, M.; Sloan, A.J.; Major, P.W.; Heo, G.; Scurtescu, C.; Tsui, Y.Y.; El-Bialy, T. Effect of low-intensity pulsed ultrasound on orthodontically induced root resorption in beagle dogs. Ultrasound Med. Biol. 2014, 40, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Rego, E.B.; Inubushi, T.; Kawazoe, A.; Tanimoto, K.; Miyauchi, M.; Tanaka, E.; Takata, T.; Tanne, K. Ultrasound stimulation induces pge(2) synthesis promoting cementoblastic differentiation through ep2/ep4 receptor pathway. Ultrasound Med. Biol. 2010, 36, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, N.M.; Grainger, J.; Bader, D.L.; Knight, M.M. The potential of pulsed low intensity ultrasound to stimulate chondrocytes matrix synthesis in agarose and monolayer cultures. J. Med. Biol. Eng. Comput. 2010, 48, 1215–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buldakov, M.A.; Hassan, M.A.; Zhao, Q.L.; Feril, L.B., Jr.; Kudo, N.; Kondo, T.; Litvyakov, N.V.; Bolshakov, M.A.; Rostov, V.V.; Cherdyntseva, N.V.; et al. Influence of changing pulse repetition frequency on chemical and biological effects induced by low-intensity ultrasound in vitro. Ultrason. Sonochem. 2009, 16, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jiang, X.; Du, K.; Cai, Q. Effects of Low-Intensity Ultrasound on Cell Proliferation and Reproductivity. Trans. Tianjin Univ. 2016, 22, 125–131. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Yarmolenko, P.S.; Moon, E.J.; Landon, C.; Manzoor, A.; Hochman, D.W.; Viglianti, B.L.; Dewhirst, M.W. Thresholds for thermal damage to normal tissues: An update. Int. J. Hyperth. 2011, 27, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, A. Voltage to pressure conversion: Are you getting ‘phased’ by the problem? J. Phys. 2004, 1, 57–62. [Google Scholar] [CrossRef]
- Nightingale, K.R.; Church, C.C.; Harris, G.; Wear, K.A.; Bailey, M.R.; Carson, P.L.; Jiang, H.; Sandstrom, K.L.; Szabo, T.L.; Ziskin, M.C. Conditionally Increased Acoustic Pressures in Nonfetal Diagnostic Ultrasound Examinations Without Contrast Agents: A Preliminary Assessment. J. Ultrasound Med. 2015, 34, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, G. Therapeutic ultrasound. Eur. J. Ultrasound 1999, 9, 3–9. [Google Scholar] [CrossRef]
- Draper, D.O.; Castel, J.C.; Castel, D. Rate of temperature increase in human muscle during 1 mhz and 3 mhz continuous ultrasound. J. Orthop. Sports Phys. Ther. 1995, 22, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, S.; Sclabas, G.M.; Schmidt, C.; Frederick, W.A.; Dong, Q.G.; Abbruzzese, J.L.; Evans, D.B.; Baker, C.; Chiao, P.J. Function of nuclear factor kappab in pancreatic cancer metastasis. Clin. Cancer Res. 2003, 9, 346–354. [Google Scholar] [PubMed]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Ohashi, T.; Haruki, K.; Fujiwara, Y.; Iida, T.; Shiba, H.; Uwagawa, T.; Kobayashi, H.; Yanaga, K. Combination treatment using adenovirus vector-mediated tumor necrosis factor-alpha gene transfer and a NF-kappaB inhibitor for pancreatic cancer in mice. Cancer Lett. 2011, 306, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.R.; King, C.R.; Osborn, R.; Fairweather, W.R.; O’Reilly, E.M.; Thornton, M.O.; Wei, L.L. Combination of human tumor necrosis factor-alpha (hTNF-alpha) gene delivery with gemcitabine is effective in models of pancreatic cancer. Cancer Gene Ther. 2009, 16, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Egberts, J.H.; Cloosters, V.; Noack, A.; Schniewind, B.; Thon, L.; Klose, S.; Kettler, B.; von Forstner, C.; Kneitz, C.; Tepel, J.; et al. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2008, 68, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.J.; Schmidt-Strassburger, U.; Huber, M.A.; Wiedemann, E.M.; Beug, H.; Wirth, T. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett. 2010, 295, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Park, S.; Phillips, P.A.; Santucci, N.; Goldstein, D.; Kumar, R.K.; Ramm, G.A.; Buchler, M.; Friess, H.; McCarroll, J.A.; et al. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas 2004, 29, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Bachem, M.G.; Schünemann, M.; Ramadani, M.; Siech, M.; Beger, H.; Buck, A.; Zhou, S.; Schmid-Kotsas, A.; Adler, G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 2005, 128, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Muerkoster, S.; Wegehenkel, K.; Arlt, A.; Witt, M.; Sipos, B.; Kruse, M.L.; Sebens, T.; Klöppel, G.; Kalthoff, H.; Fölsch, U.R.; et al. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res. 2004, 64, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Baumert, J.T.; Sparmann, G.; Emmrich, J.; Liebe, S.; Jaster, R. Inhibitory effects of interferons on pancreatic stellate cell activation. World J. Gastroenterol. 2006, 12, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Nukui, Y.; Picozzi, V.J.; Traverso, L.W. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am. J. Surg. 2000, 179, 367–371. [Google Scholar] [CrossRef]
- Picozzi, V.J.; Kozarek, R.A.; Traverso, L.W. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am. J. Surg. 2003, 185, 476–480. [Google Scholar] [CrossRef]
- Landvik, N.E.; Hart, K.; Skaug, V.; Stangeland, L.B.; Haugen, A.; Zienolddiny, S. A specific interleukin-1B haplotype correlates with high levels of IL1B mRNA in the lung and increased risk of non-small cell lung cancer. Carcinogenesis 2009, 30, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Aksentijevich, I.; Masters, S.L.; Ferguson, P.J.; Dancey, P.; Frenkel, J.; Van Royen-Kerkhoff, A.; Laxer, R.; Tedgård, U.; Cowen, E.W.; Pham, T.H.; et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N. Engl. J. Med. 2009, 360, 2426–2437. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Carmi, Y.; Apte, R.N. The role IL-1 in tumor-mediated angiogenesis. Front. Physiol. 2014, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Angst, E.; Reber, H.A.; Hines, O.J.; Eibl, G. Mononuclear cell-derived interleukin-1 beta confers chemoresistance in pancreatic cancer cells by upregulation of cyclooxygenase-2. Surgery 2008, 144, 57–65. [Google Scholar] [CrossRef] [PubMed]



| Time of Ultrasound Application (min) | Temperature (°C) |
|---|---|
| 0 | 20.1 |
| 5 | 20.2 |
| 10 | 20.2 |
| 30 | 20.3 |
| 60 | 20.6 |
| 120 | 21.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazou, D.; Maimon, N.; Munn, L.L.; Gonzalez, I. Effects of Low Intensity Continuous Ultrasound (LICU) on Mouse Pancreatic Tumor Explants. Appl. Sci. 2017, 7, 1275. https://doi.org/10.3390/app7121275
Bazou D, Maimon N, Munn LL, Gonzalez I. Effects of Low Intensity Continuous Ultrasound (LICU) on Mouse Pancreatic Tumor Explants. Applied Sciences. 2017; 7(12):1275. https://doi.org/10.3390/app7121275
Chicago/Turabian StyleBazou, Despina, Nir Maimon, Lance L. Munn, and Iciar Gonzalez. 2017. "Effects of Low Intensity Continuous Ultrasound (LICU) on Mouse Pancreatic Tumor Explants" Applied Sciences 7, no. 12: 1275. https://doi.org/10.3390/app7121275
APA StyleBazou, D., Maimon, N., Munn, L. L., & Gonzalez, I. (2017). Effects of Low Intensity Continuous Ultrasound (LICU) on Mouse Pancreatic Tumor Explants. Applied Sciences, 7(12), 1275. https://doi.org/10.3390/app7121275