Effect of the Nature and Relative Concentration of Substrate, Water Mineralization, and Storage Temperature on the Oxidants Produced by Lactoperoxidase and on Their Antifungal Activity against Penicillium expansum and Botrytis cinerea
Abstract
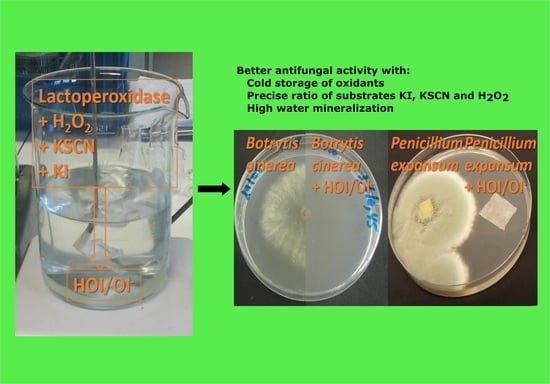
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Oxidant Production
2.3. Water Mineral Characteristics
2.4. Penicillium Expansum and Botrytis Cinerea Cultures
2.5. Oxidants Colorimetric Dosage
2.6. Ion Chromatography
2.7. 13C Nuclear Magnetic Resonance Measurements
2.8. In Vitro Antifungal Tests
3. Results
3.1. Influence of the Nature of the Substrates on the Oxidants and on Their Antifungal Activity
3.2. Influence of Water Mineralization on Oxidant Production
3.3. Influence of the Storage Temperature on Oxidant Stability and Antifungal Activity
3.4. Influence of the Relative Substrate Concentrations on Oxidant Levels and Antifungal Activity
3.4.1. Influence of the Relative Substrate Concentrations on Oxidant Levels
3.4.2. Influence of the Substrate Concentrations on Antifungal Activity
4. Discussion
4.1. Nature of the Reaction Product
4.2. Influence of Water Mineralization
4.3. Influence of the Storage Temperature on the Stability and Antifungal Activity of the Oxidant
4.4. Influence of the Relative Substrate Concentrations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jijakli, M.H.; Lepoivre, P. State of the Art and Challenges of Post-harvest Disease Management in Apples. In Disease Management in Fruits and Vegetables; Mukerij, K.G., Ed.; Springer Netherlands: Cham, The Netherlands, 2004; Volume 1, pp. 59–94. [Google Scholar]
- Snini, S.P.; Tannous, J.; Heuillard, P.; Bailly, S.; Lippi, Y.; Zehraoui, E.; Barreau, C.; Oswald, I.P.; Puel, O. Patulin is a cultivar-dependent aggressiveness factor favouring the colonization of apples by Penicillium expansum. Mol. Plant Pathol. 2016, 17, 920–930. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Reverberi, M.; Punelli, M.; Ippolito, A.; Fanelli, C. Study on the role of patulin on pathogenicity and virulence of Penicillium expansum. Int. J. Food Microbiol. 2012, 153, 323–331. [Google Scholar] [CrossRef]
- ANSES. Étude Nationale de Surveillance des Expositions Alimentaires aux Substances Chimiques—2e Étude de L’alimentation Totale 2006–2010 (EAT 2). Tome 1: Contaminants Inorganiques, Minéraux, Polluants Organiques Persistants, Mycotoxines et Phyto-Estrogènes; ANSES: Buenos Aires, Argentina, 2011.
- Tenovuo, J.; Makinen, K.K.; Sievers, G. Antibacterial effect of lactoperoxidase and myeloperoxidase against Bacillus cereus. Antimicrob. Agents Chemother. 1985, 27, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Bjorck, L.; Claesson, O. Correlation between concentration of hypothiocyanate and antibacterial effect of the lactoperoxidase system against Escherichia coli. J. Dairy Sci. 1980, 63, 919–922. [Google Scholar] [CrossRef]
- Oram, J.D.; Reiter, B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The effect of the inhibitory system on susceptible and resistant strains of group N streptococci. Biochem. J. 1966, 100, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bafort, F.; Parisi, O.; Perraudin, J.-P.; Jijakli, M.H. Mode of Action of Lactoperoxidase as Related to Its Antimicrobial Activity: A Review. Enzyme Res. 2014, 2014, 517164. [Google Scholar] [CrossRef] [PubMed]
- Aune, T.M.; Thomas, E.L. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur. J. Biochem. 1977, 80, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Aune, T.M. Peroxidase-catalyzed oxidation of protein sulfhydryls mediated by iodine. Biochemistry 1977, 16, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Bafort, F.; Parisi, O.; Perraudin, J.-P.; Jijakli, M.H. The Lactoperoxidase System: A Natural Biochemical Biocontrol Agent for Pre- and Postharvest Applications. J. Phytopathol. 2017, 165, 22–34. [Google Scholar] [CrossRef]
- Pruitt, K.M.; Tenovuo, J.O. The lactoperoxidase system: Chemistry and biological significance. Immunol. Ser. 1985, 27, 96–101. [Google Scholar]
- Ahariz, M.; Courtois, P. Candida albicans susceptibility to lactoperoxidase-generated hypoiodite. Clin. Cosmet. Investig. Dent. 2010, 2, 69–78. [Google Scholar]
- Fonteh, F.A.; Grandison, A.S.; Lewis, M.J. Variations of lactoperoxidase activity and thiocyanate content in cows’ and goats’ milk throughout lactation. J. Dairy Res. 2002, 69, 401–409. [Google Scholar] [CrossRef]
- Kussendrager, K.D.; van Hooijdonk, A.C. Lactoperoxidase: Physico-chemical properties, occurrence, mechanism of action and applications. Br. J. Nutr. 2000, 84 (Suppl. 1), S19–S25. [Google Scholar] [CrossRef]
- Boots, J.W.; Floris, R. Lactoperoxidase: From catalytic mechanism to practical applications. Int. Dairy J. 2006, 16, 1272–1276. [Google Scholar] [CrossRef]
- van Hooijdonk, A.C.; Kussendrager, K.D.; Steijns, J.M. In vivo antimicrobial and antiviral activity of components in bovine milk and colostrum involved in non-specific defence. Br. J. Nutr. 2000, 84 (Suppl. 1), S127–S134. [Google Scholar] [CrossRef]
- Lenander-Lumikari, M.; Tenovuo, J.; Mikola, H. Effects of a lactoperoxidase system-containing toothpaste on levels of hypothiocyanite and bacteria in saliva. Caries Res. 1993, 27, 285–291. [Google Scholar] [CrossRef]
- Kirstila, V.; Lenander-Lumikari, M.; Soderling, E.; Tenovuo, J. Effects of oral hygiene products containing lactoperoxidase, lysozyme, and lactoferrin on the composition of whole saliva and on subjective oral symptoms in patients with xerostomia. Acta Odontol. Scand. 1996, 54, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.A.; Guardia-Lopez, I.; Gonzalez-Moles, M.A. Evaluation of the clinical efficacy of a mouthwash and oral gel containing the antimicrobial proteins lactoperoxidase, lysozyme and lactoferrin in elderly patients with dry mouth—A pilot study. Gerodontology 2008, 25, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ihalin, R.; Loimaranta, V.; Lenander-Lumikari, M.; Tenovuo, J. The effects of different (pseudo)halide substrates on peroxidase-mediated killing of Actinobacillus actinomycetemcomitans. J. Periodontal Res. 1998, 33, 421–427. [Google Scholar] [CrossRef]
- Bafort, F.; Barthelemy, J.-P.; Parisi, O.; Perraudin, J.-P.; Jijakli, H. Development of a colorimetric method for the dosage of OI− anions and I2 in aqueous media. Commun. Agric. Appl. Biol. Sci. 2014, 79, 155–160. [Google Scholar] [PubMed]
- WHO; UNICEF; ICCIDD. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. A Guide for Programme Managers; WHO Press: Geneva, Switzerland, 2007. [Google Scholar]
- Nagy, P.; Alguindigue, S.S.; Ashby, M.T. Lactoperoxidase-catalyzed oxidation of thiocyanate by hydrogen peroxide: A reinvestigation of hypothiocyanite by nuclear magnetic resonance and optical spectroscopy. Biochemistry 2006, 45, 12610–12616. [Google Scholar] [CrossRef] [PubMed]
- Bafort, F.; Damblon, C.; Smargiasso, N.; De Pauw, E.; Perraudin, J.-P.; Jijakli, M.H. Reaction product variability and biological activity of the lactoperoxidase system depending on medium ionic strength and pH, and on substrate relative concentration. Chem. Biodivers. 2018, 15, e170049. [Google Scholar] [CrossRef] [PubMed]
- Arlandson, M.; Decker, T.; Roongta, V.A.; Bonilla, L.; Mayo, K.H.; MacPherson, J.C.; Hazen, S.L.; Slungaard, A. Eosinophil peroxidase oxidation of thiocyanate. Characterization of major reaction products and a potential sulfhydryl-targeted cytotoxicity system. J. Biol. Chem. 2001, 276, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Bosch, E.H.; van Doorne, H.; de Vries, S. The lactoperoxidase system: The influence of iodide and the chemical and antimicrobial stability over the period of about 18 months. J. Appl. Microbiol. 2000, 89, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Schlorke, D.; Flemmig, J.; Birkemeyer, C.; Arnhold, J. Formation of cyanogen iodide by lactoperoxidase. J. Inorg. Biochem. 2016, 154, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sebaa, S.; Faltot, M.; De Breucker, S.; Boucherit-Otmani, Z.; Bafort, F.; Perraudin, J.-P.; Courtois, P. Ex vivo decontamination of yeast-colonized dentures by iodine–thiocyanate complexes. Clin. Cosmet. Investig. Dent. 2018, 10, 149–158. [Google Scholar] [CrossRef]
- Aune, T.M.; Thomas, E.L. Oxidation of protein sulfhydryls by products of peroxidase-catalyzed oxidation of thiocyanate ion. Biochemistry 1978, 17, 1005–1010. [Google Scholar] [CrossRef]
- Thomas, E.L.; Aune, T.M. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: Correlation of sulfhydryl oxidation with antimicrobial action. Infect. Immun. 1978, 20, 456–463. [Google Scholar]
- Thomas, E.L.; Aune, T.M. Oxidation of Escherichia coli sulfhydryl components by the peroxidase-hydrogen peroxide-iodide antimicrobial system. Antimicrob. Agents Chemother. 1978, 13, 1006–1010. [Google Scholar] [CrossRef]
- Prütz, W.A.; Kissner, R.; Nauser, T.; Koppenol, W.H. On the oxidation of cytochrome c by hypohalous acids. Arch. Biochem. Biophys. 2001, 389, 110–122. [Google Scholar] [CrossRef]
- Prütz, W.A.; Kissner, R.; Koppenol, W.H.; Rüegger, H. On the irreversible destruction of reduced nicotinamide nucleotides by hypohalous acids. Arch. Biochem. Biophys. 2000, 380, 181–191. [Google Scholar] [CrossRef]
- Ashby, M.T. Chapter 8—Hypothiocyanite. Adv. Inorg. Chem. 2012, 64, 263–303. [Google Scholar]
- Magnusson, R.P.; Taurog, A.; Dorris, M.L. Mechanism of iodide-dependent catalatic activity of thyroid peroxidase and lactoperoxidase. J. Biol. Chem. 1984, 259, 197–205. [Google Scholar]
- Ferrari, R.P.; Ghibaudi, E.M.; Traversa, S.; Laurenti, E.; De Gioia, L.; Salmona, M. Spectroscopic and binding studies on the interaction of inorganic anions with lactoperoxidase. J. Inorg. Biochem. 1997, 68, 17–26. [Google Scholar] [CrossRef]
- Long, C.; Skoog, D.A. A Thiocyanate Complex of Iodine(I). Inorg. Chem. 1966, 5, 206–210. [Google Scholar] [CrossRef]
- Gottardi, W. Iodine and disinfection: Theoretical study on mode of action, efficiency, stability, and analytical aspects in the aqueous system. Arch. Pharm. 1999, 332, 151–157. [Google Scholar] [CrossRef]
- Bichsel, Y.; Von Gunten, U. Oxidation of iodide and hypoiodous acid in the disinfection of natural waters. Environ. Sci. Technol. 1999, 33, 4040–4045. [Google Scholar] [CrossRef]
- Bichsel, Y.; Von Gunten, U. Hypoiodous acid: Kinetics of the buffer-catalyzed disproportionation. Water Res. 2000, 34, 3197–3203. [Google Scholar] [CrossRef]
- Holm, K.A. Automated determination of microbial peroxidase activity in fermentation samples using hydrogen peroxide as the substrate and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) as the electron donor in a flow injection system. Analyst 1995, 120, 2101–2105. [Google Scholar] [CrossRef]
- Marcozzi, G.; Di Domenico, C.; Spreti, N. Effects of surfactants on the stabilization of the bovine lactoperoxidase activity. Biotechnol. Prog. 1998, 14, 653–656. [Google Scholar] [CrossRef]
- Arnhold, J.; Monzani, E.; Furtmüller, P.G.; Zederbauer, M.; Casella, L.; Obinger, C. Kinetics and thermodynamics of halide and nitrite oxidation by mammalian heme peroxidases. Eur. J. Inorg. Chem. 2006, 19, 3801–3811. [Google Scholar] [CrossRef]
- Battistuzzi, G.; Bellei, M.; Vlasits, J.; Banerjee, S.; Furtmüller, P.G.; Sola, M.; Obinger, C. Redox thermodynamics of lactoperoxidase and eosinophil peroxidase. Arch. Biochem. Biophys. 2010, 494, 72–77. [Google Scholar] [CrossRef]
- Furtmüller, P.G.; Arnhold, J.; Jantschko, W.; Zederbauer, M.; Jakopitsch, C.; Obinger, C. Standard reduction potentials of all couples of the peroxidase cycle of lactoperoxidase. J. Inorg. Biochem. 2005, 99, 1220–1229. [Google Scholar] [CrossRef]






| Minerals Content mg/L | Low Mineralization Water | Medium Low Mineralization Water | Medium High Mineralization Water | High Mineralization Water |
|---|---|---|---|---|
| pH | Cristaline Spring: Cristal-Roc | Tap Water | Vittel | Contrex |
| Ca++ | 73 | 131 | 240 | 468 |
| Mg++ | 2 | 18 | 42 | 74.5 |
| Na+ | 4.5 | 32 | 5 | 9.4 |
| K+ | 1.3 | 2.1 | 1.9 | 2.8 |
| HCO3− | 200 | 366 | 384 | 372 |
| SO4− | 20 | 84 | 400 | 1121 |
| NO3− | <1 | 40 | 4.4 | 2.9 |
| Cl− | 10 | 36 | 8 | 7.6 |
| Dry residue | 223 | 550 | 1084 | 2078 |
| pH | 7.7 | 7.4 | 7.6 | 7.4 |
| Substrate Doses | |||
|---|---|---|---|
| 1.2 mM KSCN + 1.2 mM H2O2 | 1.2 mM KSCN + 5.4 mM KI + 6.6 mM H2O2 | 5.4 mM KI + 5.4 mM H2O2 | |
| Storage (days) | P. expansum inhibition (%) | ||
| 0 | 0 ± 4 ns | 94 ± 3 a | 0 ± 1 ns |
| 1 | - | 94 ± 8 a | - |
| 2 | - | 90 ± 3 a | - |
| 3 | - | 86 ± 3 a | - |
| 5 | - | 94 ± 5 a | - |
| 10 | - | 80 ± 5 a | - |
| Oxidant Concentration (µM) | |||
| 0 | 359 ± 24 | 405 ± 80 | 54 ± 6 |
| 1 | 72 ± 9 | 381 ± 28 | 55 ± 4 |
| 2 | 17 ± 7 | 361 ± 34 | 52 ± 4 |
| 3 | 10 ± 5 | 362 ± 28 | 45 ± 6 |
| 5 | 0 ± 0 | 277 ± 50 | 49 ± 11 |
| 10 | 0 ± 0 | 263 ± 28 | 52 ± 9 |
| Water Mineralization | ||||
|---|---|---|---|---|
| Low | Medium Low | Medium High | High | |
| Substrate Doses | Oxidant Concentration (µM) | |||
| Half-dose | 291 ± 20 | 254 ± 44 | 235 ± 32 | 256 ± 16 |
| Full-dose | 383 ± 25 | 407 ± 17 | 503 ± 33 | 538 ± 42 |
| Double-dose | 546 ± 24 | 563 ± 18 | 785 ± 29 | 974 ± 49 |
| Storage Duration (Days) | ||||||
|---|---|---|---|---|---|---|
| 0 | 30 | 90 | 240 | 360 | ||
| Temp. | Water Mineralization | P. expansum Inhibition (%) | ||||
| 25 °C | Low | 91 ± 5 b | 81 ± 4 a | 26 ± 4 c | 0 ± 6 ns | 4 ± 2 ns |
| Medium Low | 88 ± 7 b | 24 ± 10 ns | 0 ± 2 ns | 0 ± 2 ns | 0 ± 4 ns | |
| Medium High | 92 ± 2 b | 0 ± 1 ns | 5 ± 5 ns | 0 ± 4 ns | 7 ± 7 ns | |
| High | 89 ± 3 b | 0 ± 0 ns | 0 ± 1 ns | 3 ± 6 ns | 0 ± 5 ns | |
| 4 °C | Low | 100 ± 0 a | 73 ± 8 a | 95 ± 3 a | 95 ± 3 a | 95 ± a |
| Medium Low | 99 ± 1 a | 89 ± 5 a | 91 ± 2 a | 96 ± 4 a | 92 ± a | |
| Medium High | 100 ± 0 a | 73 ± 7 a | 53 ± 3 bc | 61 ± c | 90 ± a | |
| High | 100 ± 0 a | 75 ± 4 a | 63 ± 11 b | 85 ± b | 90 ± a | |
| Storage Duration (Days) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 90 | 150 | 240 | 300 | 360 | ||
| Temp. | Water Mineral. | P. expansum Inhibition (%) | ||||||
| 25 °C | Low | 89 ± 2 b | 98 ± 1 ab | 10 ± 7 ns | 14 ± 10 ns | 17 ± 11 ns | 6 ± 5 ns | 2 ± 1 ns |
| Medium Low | 89 ± 1 b | 96 ± 2 ab | 8 ± 5 ns | 3 ± 2 ns | 15 ± 8 ns | 2 ± 1 ns | 4 ± 3 ns | |
| Medium High | 92 ± 0 ab | 95 ± 4 ab | 90 ± 4 a | 85 ± 4 a | 2 ± 1 ns | 0 ± 0 ns | 5 ± 4 ns | |
| High | 95 ± 0 a | 91 ± 5 a | 93 ± 5 a | 86 ± 5 ab | 89 ± 6 a | 1 ± 1 ns | 1 ± 1 ns | |
| 4 °C | Low | 90 ± 5 ab | 97 ± 2 ab | 100 ± 0 a | 98 ± 3 b | 92 ± 5 a | 93 ± 4 a | 94 ± 5 a |
| Medium Low | 90 ± 1 ab | 99 ± 1 b | 92 ± 3 a | 89 ± 5 ab | 89 ± 5 a | 92 ± 1 a | 92 ± 6 a | |
| Medium High | 91 ± 3 ab | 93 ± 3 ab | 87 ± 0 a | 83 ± 5 a | 89 ± 4 a | 92 ± 0 a | 91 ± 4 a | |
| High | 94 ± 1 ab | 94 ± 3 ab | 88 ± 2 a | 85 ± 6 a | 90 ± 6 a | 92 ± 3 a | 92 ± 5 a | |
| Storage Duration (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 90 | 120 | 180 | 240 | 300 | 360 | ||
| Temp. | Water Mineral. | P. expansum Inhibition (%) | |||||||
| 25 °C | Low | 94 ± 3 a | 14 ± 10 ns | 0 ± 0 ns | 10 ± 4 ns | 0 ± 1 ns | 0 ± 0 ns | 2 ± 1 ns | 0 ± 0 ns |
| M. Low | 93 ± 4 a | 0 ± 0 ns | 0 ± 0 ns | 8 ± 2 ns | 6 ± 2 ns | 2 ± 1 ns | 5 ± 3 ns | 3 ± 2 ns | |
| M. High | 91 ± 2 a | 88 ± 8 a | 0 ± 0 ns | 4 ± 4 ns | 5 ± 3 ns | 9 ± 4 ns | 4 ± 2 ns | 2 ± 1 ns | |
| High | 91 ± 4 a | 87 ± 5 a | 88 ± 1 a | 0 ± 0 ns | 0 ± 0 ns | 3 ± 3 ns | 0 ± 1 ns | 0 ± 0 ns | |
| 4 °C | Low | 99 ± 1 b | 88 ± 4 a | 90 ± 0 a | 91 ± 1 a | 88 ± 3 a | 94 ± 1 a | 31 ± 10 a | 59 ± 5 a |
| M. Low | 98 ± 2 b | 89 ± 4 a | 89 ± 3 a | 91 ± 2 a | 87 ± 2 a | 6 ± 3 ns | 1 ± 1 ns | 10 ± 3 ns | |
| M. High | 99 ± 0 b | 86 ± 3 a | 88 ± 3 a | 92 ± 2 a | 85 ± 4 a | 92 ± 2 a | 90 ± 5 b | 89 ± 4 b | |
| High | 98 ± 2 b | 90 ± 6 a | 88 ± 4 a | 90 ± 3 a | 84 ± 3 a | 91 ± 0 a | 97 ± 4 b | 92 ± 5 b | |
| [KI + KSCN] (mM) | KI/KSCN Ratio | [KI-KSCN-H2O2] (mM) | Dilution Factor | B. cinerea Inhibition (%) |
|---|---|---|---|---|
| Influence of the total [KI + KSCN] concentration | ||||
| 6.6 mM | 4.5 | 5.4-1.2-6.6 | 1:3 | 88 ± 5 |
| 5.4-1.2-6.6 | 1:5 | 84 ± 4 | ||
| 5.4-1.2-6.6 | 1:10 | 83 ± 2 | ||
| 4.4 mM | 4.5 | 3.6-0.8-3.6 | 1:3 | 88 ± 5 |
| 3.6-0.8-3.6 | 1:5 | 88 ± 6 | ||
| 3.6-0.8-3.6 | 1:10 | 70 ± 11 | ||
| 3.3 mM | 4.5 | 2.7-0.6-3.3 | 1:3 | 88 ± 6 |
| 2.7-0.6-3.3 | 1:5 | 83 ± 10 | ||
| 2.7-0.6-3.3 | 1:10 | 34 ± 1 | ||
| 5.6 mM | 7.84 | 5-0.64-5.64 | 1:3 | 92 ± 0 |
| 5-0.64-5.64 | 1:5 | 69 ± 11 | ||
| 5-0.64-5.64 | 1:10 | 42 ± 6 | ||
| 3.95 mM | 7.84 | 3.5-0.45-3.95 | 1:3 | 71 ± 11 |
| 3.5-0.45-3.95 | 1:5 | 42 ± 5 | ||
| 3.5-0.45-3.95 | 1:10 | 32 ± 8 | ||
| 2.25 mM | 7.84 | 2-0.25-2.25 | 1:3 | 38 ± 0 |
| 2-0.25-2.25 | 1:5 | 39 ± 6 | ||
| 2-0.25-2.25 | 1:10 | 29 ± 7 | ||
| Influence of the KI/KSCN ratio | ||||
| 1.12 mM | 2.3 | 0.78-0.34-1.2 | 1:3 | 90 ± 4 |
| 0.78-0.34-1.2 | 1:5 | 25 ± 0 | ||
| 0.78-0.34-1.2 | 1:10 | 22 ± 0 | ||
| 3.9 mM | 2.25 | 2.7-1.2-3.9 | 1:3 | 84 ± 1 |
| 2.7-1.2-3.9 | 1:5 | 81 ± 2 | ||
| 2.7-1.2-3.9 | 1:10 | 24 ± 7 | ||
| 2.95 mM | 2.25 | 1.35-0.6-1.9 | 1:3 | 87 ± 3 |
| 1.35-0.6-1.9 | 1:5 | 52 ± 4 | ||
| 1.35-0.6-1.9 | 1:10 | 20 ± 12 | ||
| 2.4 mM | 1.1 | 1.35-1.2-2.5 | 1:3 | 28 ± 4 |
| 1.35-1.2-2.5 | 1:5 | 31 ± 4 | ||
| 1.35-1.2-2.5 | 1:10 | 26 ± 5 | ||
| 1.3 mM | 1.1 | 0.67-0.6-1.27 | 1:3 | 16 ± 6 |
| 0.67-0.6-1.27 | 1:5 | 17 ± 6 | ||
| 0.67-0.6-1.27 | 1:10 | 13 ± 2 | ||
| Influence of the H2O2 concentration | ||||
| 2.64 mM | 4.5 | 2.16-0.48-0.5 | 1:3 | 10 ± 7 |
| 2.16-0.48-0.5 | 1:5 | 12 ± 3 | ||
| 2.16-0.48-0.5 | 1:10 | 20 ± 2 | ||
| 2.64 mM | 4.5 | 2.16-0.48-1.1 | 1:3 | 39 ± 3 |
| 2.16-0.48-1.1 | 1:5 | 10 ± 9 | ||
| 2.16-0.48-1.1 | 1:10 | 7 ± 7 | ||
| 2.64 mM | 4.5 | 2.16-0.48-2.7 | 1:3 | 80 ± 3 |
| 2.16-0.48-2.7 | 1:5 | 45 ± 5 | ||
| 2.16-0.48-2.7 | 1:10 | 14 ± 1 | ||
| T1/2 (Days) | ||||||
|---|---|---|---|---|---|---|
| 25 °C | 4 °C | 25 °C | 4 °C | 25 °C | 4 °C | |
| Water Mineralization | Double-Dose | Full-Dose | Half-Dose | |||
| Low | 11 | 34 | 49 | 309 | 109 | 494 |
| Medium Low | 15 | 119 | 36 | 248 | 100 | 464 |
| Medium High | 20 | 165 | 98 | 504 | 73 | 433 |
| High | 33 | 229 | 121 | 491 | 94 | 529 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bafort, F.; Damblon, C.; Lognay, G.C.; Barthelemy, J.-P.; Perraudin, J.-P.; Jijakli, M.H. Effect of the Nature and Relative Concentration of Substrate, Water Mineralization, and Storage Temperature on the Oxidants Produced by Lactoperoxidase and on Their Antifungal Activity against Penicillium expansum and Botrytis cinerea. Appl. Sci. 2019, 9, 197. https://doi.org/10.3390/app9010197
Bafort F, Damblon C, Lognay GC, Barthelemy J-P, Perraudin J-P, Jijakli MH. Effect of the Nature and Relative Concentration of Substrate, Water Mineralization, and Storage Temperature on the Oxidants Produced by Lactoperoxidase and on Their Antifungal Activity against Penicillium expansum and Botrytis cinerea. Applied Sciences. 2019; 9(1):197. https://doi.org/10.3390/app9010197
Chicago/Turabian StyleBafort, Françoise, Christian Damblon, Georges C. Lognay, Jean-Paul Barthelemy, Jean-Paul Perraudin, and Mohamed Haïssam Jijakli. 2019. "Effect of the Nature and Relative Concentration of Substrate, Water Mineralization, and Storage Temperature on the Oxidants Produced by Lactoperoxidase and on Their Antifungal Activity against Penicillium expansum and Botrytis cinerea" Applied Sciences 9, no. 1: 197. https://doi.org/10.3390/app9010197
APA StyleBafort, F., Damblon, C., Lognay, G. C., Barthelemy, J. -P., Perraudin, J. -P., & Jijakli, M. H. (2019). Effect of the Nature and Relative Concentration of Substrate, Water Mineralization, and Storage Temperature on the Oxidants Produced by Lactoperoxidase and on Their Antifungal Activity against Penicillium expansum and Botrytis cinerea. Applied Sciences, 9(1), 197. https://doi.org/10.3390/app9010197