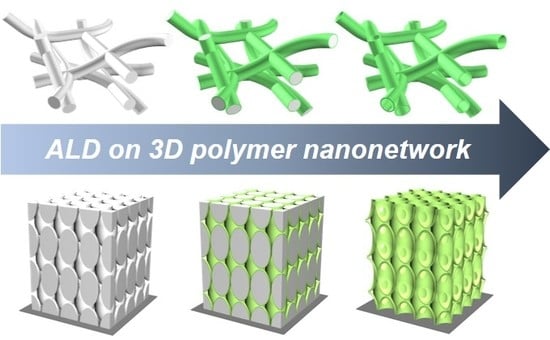
Atomic Layer Deposition of Inorganic Thin Films on 3D Polymer Nanonetworks
Abstract
:1. Introduction
2. Preparation of 3D Polymer Nanonetworks
3. Conformal Deposition of Inorganic Thin Films on 3D Polymer Nanonetworks
4. Application of 3D Inorganic Nanonetworks
4.1. Organic–Inorganic Hybrid Nanocomposites
4.2. Highly Porous Hollow Inorganics
5. Summary and Outlook
Funding
Conflicts of Interest
References
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Ritala, M.; Leskela, M. Handbook of thin film materials. Depos. Process. Thin Film 2002, 1, 103. [Google Scholar]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
- Hwang, C.S. Atomic Layer Deposition for Semiconductors; Springer: Berlin, Germany, 2013. [Google Scholar]
- Kim, H. Atomic layer deposition of metal and nitride thin films: Current research efforts and applications for semiconductor device processing. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2003, 21, 2231–2261. [Google Scholar] [CrossRef]
- Groner, M.D.; Fabreguette, F.H.; Elam, J.W.; George, S.M. Low-Temperature Al2O3 Atomic Layer Deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Knez, M.; Nielsch, K.; Niinistö, L. Synthesis and Surface Engineering of Complex Nanostructures by Atomic Layer Deposition. Adv. Mater. 2007, 19, 3425–3438. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Vaynzof, Y.; Sepe, A.; Guldin, S.; Scherer, M.; Cunha, P.; Roth, S.V.; Steiner, U. Gyroid-Structured 3D ZnO Networks Made by Atomic Layer Deposition. Adv. Funct. Mater. 2014, 24, 863–872. [Google Scholar] [CrossRef]
- Ma, W.-C.; Huang, W.-S.; Ku, C.-S.; Ho, R.-M. Nanoporous gyroid metal oxides with controlled thickness and composition by atomic layer deposition from block copolymer templates. J. Mater. Chem. C 2016, 4, 840–849. [Google Scholar] [CrossRef]
- Werner, J.G.; Scherer, M.R.J.; Steiner, U.; Wiesner, U. Gyroidal mesoporous multifunctional nanocomposites via atomic layer deposition. Nanoscale 2014, 6, 8736–8742. [Google Scholar] [CrossRef]
- Li, F.; Yao, X.; Wang, Z.; Xing, W.; Jin, W.; Huang, J.; Wang, Y. Highly Porous Metal Oxide Networks of Interconnected Nanotubes by Atomic Layer Deposition. Nano Lett. 2012, 12, 5033–5038. [Google Scholar] [CrossRef]
- Guo, L.; Zhong, Z.; Wang, Y. Atomic Layer Deposition on Block Copolymer Membranes with Gyroidal Nanopores Toward Periodically Nanostructured Vapor Sensors: Nanotubes versus Nanorods. Adv. Mater. Interfaces 2016, 3, 1600017. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, R.; Chen, V.H.; Khabiboulline, E.T.; Braun, P.; Atwater, H.A. Three-Dimensional Single Gyroid Photonic Crystals with a Mid-Infrared Bandgap. ACS Photonics 2016, 3, 1131–1137. [Google Scholar] [CrossRef] [Green Version]
- Frölich, A.; Wegener, M. Spectroscopic characterization of highly doped ZnO films grown by atomic-layer deposition for three-dimensional infrared metamaterials [Invited]. Opt. Mater. Express 2011, 1, 883–889. [Google Scholar] [CrossRef]
- Jang, D.; Meza, L.R.; Greer, F.; Greer, J.R. Fabrication and deformation of three-dimensional hollow ceramic nanostructures. Nat. Mater. 2013, 12, 893–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meza, L.R.; Das, S.; Greer, J.R. Strong, lightweight, and recoverable three-dimensional ceramic nanolattices. Science 2014, 345, 1322–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawata, S.; Sun, H.-B.; Tanaka, T.; Takada, K. Finer features for functional microdevices. Nature 2001, 412, 697–698. [Google Scholar] [CrossRef]
- Brzezinski, A.; Chen, Y.-C.; Wiltzius, P.; Braun, P.V. Complex three-dimensional conformal surfaces formed by atomic layer deposition: Computation and experimental verification. J. Mater. Chem. 2009, 19, 9126–9130. [Google Scholar] [CrossRef]
- Ramanan, V.; Nelson, E.; Brzezinski, A.; Braun, P.V.; Wiltzius, P. Three dimensional silicon-air photonic crystals with controlled defects using interference lithography. Appl. Phys. Lett. 2008, 92, 173304. [Google Scholar] [CrossRef]
- King, J.S.; Graugnard, E.; Roche, O.M.; Sharp, D.N.; Scrimgeour, J.; Denning, R.G.; Turberfield, A.J.; Summers, C.J. Infiltration and Inversion of Holographically Defined Polymer Photonic Crystal Templates by Atomic Layer Deposition. Adv. Mater. 2006, 18, 1561–1565. [Google Scholar] [CrossRef]
- Ahn, C.; Park, J.; Kim, D.; Jeon, S. Monolithic 3D titania with ultrathin nanoshell structures for enhanced photocatalytic activity and recyclability. Nanoscale 2013, 5, 10384–10389. [Google Scholar] [CrossRef]
- Cho, S.; Ahn, C.; Park, J.; Jeon, S. 3D nanostructured N-doped TiO2 photocatalysts with enhanced visible absorption. Nanoscale 2018, 10, 9747–9751. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, K.-I.; Kim, K.; Kim, D.-C.; Cho, D.; Lee, J.H.; Jeon, S. Rapid, High-Resolution 3D Interference Printing of Multilevel Ultralong Nanochannel Arrays for High-Throughput Nanofluidic Transport. Adv. Mater. 2015, 27, 8000–8006. [Google Scholar] [CrossRef]
- Bong, J.; Ahn, C.; Lim, T.; Park, J.H.; Kwak, S.K.; Jeon, S.; Ju, S. Controlled three-dimensional interconnected capillary structures for liquid repellency engineering. RSC Adv. 2016, 6, 61909–61914. [Google Scholar] [CrossRef]
- Na, Y.-E.; Shin, D.; Kim, K.; Ahn, C.; Jeon, S.; Jang, D. Emergence of New Density–Strength Scaling Law in 3D Hollow Ceramic Nanoarchitectures. Small 2018, 14, 1802239. [Google Scholar] [CrossRef]
- Ahn, C.; Kim, S.-M.; Jung, J.-W.; Park, J.; Kim, T.; Lee, S.E.; Jang, D.; Hong, J.-W.; Han, S.M.; Jeon, S. Multifunctional Polymer Nanocomposites Reinforced by 3D Continuous Ceramic Nanofillers. ACS Nano 2018, 12, 9126–9133. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, J.; Hong, S.; Park, S.H.; Jeon, S.G.; Ahn, C.; Song, J.Y.; Jeon, S. Anomalous thermoelectricity of pure ZnO from 3D continuous ultrathin nanoshell structures. Nanoscale 2018, 10, 3046–3052. [Google Scholar] [CrossRef]
- Bagal, A.; Zhang, X.A.; Shahrin, R.; Dandley, E.C.; Zhao, J.; Poblete, F.R.; Oldham, C.J.; Zhu, Y.; Parsons, G.N.; Bobko, C.; et al. Large-Area Nanolattice Film with Enhanced Modulus, Hardness, and Energy Dissipation. Sci. Rep. 2017, 7, 9145. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.A.; Bagal, A.; Dandley, E.C.; Zhao, J.; Oldham, C.J.; Wu, B.-I.; Parsons, G.N.; Chang, C.-H. Ordered 3D Thin-Shell Nanolattice Materials with Near-Unity Refractive Indices. Adv. Funct. Mater. 2015, 25, 6644–6649. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, H.; Ahn, C.; Park, J.; Jeon, S. Strategies to improve the photocatalytic activity of TiO2: 3D nanostructuring and heterostructuring with graphitic carbon nanomaterials. Nanoscale 2019, 11, 7025–7040. [Google Scholar] [CrossRef]
- Moon, J.H.; Yang, S. Chemical Aspects of Three-Dimensional Photonic Crystals. Chem. Rev. 2010, 110, 547–574. [Google Scholar] [CrossRef] [PubMed]
- Kashani, H.; Ito, Y.; Han, J.; Liu, P.; Chen, M. Extraordinary tensile strength and ductility of scalable nanoporous graphene. Sci. Adv. 2019, 5, eaat6951. [Google Scholar] [CrossRef]
- Park, S.-G.; Jeon, T.Y.; Jeon, H.C.; Yang, S.-M.; Kwon, J.-D.; Mun, C.-W.; Cho, B.; Kim, C.S.; Kim, D.-H. Fabrication of 3D ZnO hollow shell structures by prism holographic lithography and atomic layer deposition. J. Mater. Chem. C 2014, 2, 1957–1961. [Google Scholar] [CrossRef]
- Campo, A.D.; Greiner, C. SU-8: A photoresist for high-aspect-ratio and 3D submicron lithography. J. Micromech. Microeng. 2007, 17, R81–R95. [Google Scholar] [CrossRef]
- Hsueh, H.-Y.; Yao, C.-T.; Ho, R.-M. Well-ordered nanohybrids and nanoporous materials from gyroid block copolymer templates. Chem. Soc. Rev. 2015, 44, 1974–2018. [Google Scholar] [CrossRef]
- Peng, Q.; Sun, X.-Y.; Spagnola, J.C.; Hyde, G.K.; Spontak, R.J.; Parsons, G.N. Atomic Layer Deposition on Electrospun Polymer Fibers as a Direct Route to Al2O3 Microtubes with Precise Wall Thickness Control. Nano Lett. 2007, 7, 719–722. [Google Scholar] [CrossRef]
- Jeon, S.; Park, J.-U.; Cirelli, R.; Yang, S.; Heitzman, C.E.; Braun, P.V.; Kenis, P.J.A.; Rogers, J.A. Fabricating complex three-dimensional nanostructures with high-resolution conformable phase masks. Proc. Natl. Acad. Sci. USA 2004, 101, 12428–12433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Kim, I.-D. Advances in Electrospun Functional Nanofibers. Macromol. Mater. Eng. 2013, 298, 473–474. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.-M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Yuan, H.; Zhou, Q.; Zhang, Y. 6—Improving fiber alignment during electrospinning. In Electrospun Nanofibers; Afshari, M., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 125–147. [Google Scholar] [CrossRef]
- Ross, C.A.; Berggren, K.K.; Cheng, J.Y.; Jung, Y.S.; Chang, J.-B. Three-Dimensional Nanofabrication by Block Copolymer Self-Assembly. Adv. Mater. 2014, 26, 4386–4396. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, H.-Y.; Chen, H.-Y.; She, M.-S.; Chen, C.-K.; Ho, R.-M.; Gwo, S.; Hasegawa, H.; Thomas, E.L. Inorganic Gyroid with Exceptionally Low Refractive Index from Block Copolymer Templating. Nano Lett. 2010, 10, 4994–5000. [Google Scholar] [CrossRef] [PubMed]
- Deubel, M.; von Freymann, G.; Wegener, M.; Pereira, S.; Busch, K.; Soukoulis, C.M. Direct laser writing of three-dimensional photonic-crystal templates for telecommunications. Nat. Mater. 2004, 3, 444. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wei, J.; Sun, Z.; Zhao, D. Large-pore ordered mesoporous materials templated from non-Pluronic amphiphilic block copolymers. Chem. Soc. Rev. 2013, 42, 4054–4070. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, D.; Schnitzler, D.; Schaefer, D.; Gottmann, J.; Kelbassa, I. Beam shaping of laser diode radiation by waveguides with arbitrary cladding geometry written with fs-laser radiation. Opt. Express 2011, 19, 25418–25425. [Google Scholar] [CrossRef] [PubMed]
- Stankevičius, E.; Daugnoraitė, E.; Račiukaitis, G. Mechanism of pillars formation using four-beam interference lithography. Opt. Lasers Eng. 2019, 116, 41–46. [Google Scholar] [CrossRef]
- Jeon, S.; Malyarchuk, V.; Rogers, J.A.; Wiederrecht, G.P. Fabricating three dimensional nanostructures using two photon lithography in a single exposure step. Opt. Express 2006, 14, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Sharp, D.N.; Harrison, M.T.; Denning, R.G.; Turberfield, A.J. Fabrication of photonic crystals for the visible spectrum by holographic lithography. Nature 2000, 404, 53–56. [Google Scholar] [CrossRef]
- Kondo, T.; Juodkazis, S.; Mizeikis, V.; Misawa, H.; Matsuo, S. Holographic lithography of periodic two-and three-dimensional microstructures in photoresist SU-8. Opt. Express 2006, 14, 7943–7953. [Google Scholar] [CrossRef]
- Jeon, T.; Kim, D.-H.; Park, S.-G. Holographic Fabrication of 3D Nanostructures. Adv. Mater. Interfaces 2018, 5, 1800330. [Google Scholar] [CrossRef]
- Ullal, C.K.; Maldovan, M.; Thomas, E.L.; Chen, G.; Han, Y.-J.; Yang, S. Photonic crystals through holographic lithography: Simple cubic, diamond-like, and gyroid-like structures. Appl. Phys. Lett. 2004, 84, 5434–5436. [Google Scholar] [CrossRef]
- Park, J.; Wang, S.; Li, M.; Ahn, C.; Hyun, J.K.; Kim, D.S.; Kim, D.K.; Rogers, J.A.; Huang, Y.; Jeon, S. Three-dimensional nanonetworks for giant stretchability in dielectrics and conductors. Nat. Commun. 2012, 3, 916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Park, J.H.; Kim, E.; Ahn, C.W.; Jang, H.I.; Rogers, J.A.; Jeon, S. Conformable Solid-Index Phase Masks Composed of High-Aspect-Ratio Micropillar Arrays and Their Application to 3D Nanopatterning. Adv. Mater. 2011, 23, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yoon, S.; Kang, K.; Jeon, S. Antireflection Behavior of Multidimensional Nanostructures Patterned Using a Conformable Elastomeric Phase Mask in a Single Exposure Step. Small 2010, 6, 1981–1985. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Seo, J.; Jung, H.K.; Hyun, G.; Park, S.Y.; Jeon, S. Direct Optical Fabrication of Fluorescent, Multilevel 3D Nanostructures for Highly Efficient Chemosensing Platforms. Adv. Funct. Mater. 2016, 26, 7170–7177. [Google Scholar] [CrossRef]
- Shir, D.J.; Jeon, S.; Liao, H.; Highland, M.; Cahill, D.G.; Su, M.F.; El-Kady, I.F.; Christodoulou, C.G.; Bogart, G.R.; Hamza, A.V.; et al. Three-Dimensional Nanofabrication with Elastomeric Phase Masks. J. Phys. Chem. B 2007, 111, 12945–12958. [Google Scholar] [CrossRef]
- Park, J.; Tahk, D.; Ahn, C.; Im, S.G.; Choi, S.-J.; Suh, K.-Y.; Jeon, S. Conformal phase masks made of polyurethane acrylate with optimized elastic modulus for 3D nanopatterning. J. Mater. Chem. C 2014, 2, 2316–2322. [Google Scholar] [CrossRef]
- Hyun, J.K.; Park, J.; Kim, E.; Lauhon, L.J.; Jeon, S. Rational Control of Diffraction and Interference from Conformal Phase Gratings: Toward High-Resolution 3D Nanopatterning. Adv. Opt. Mater. 2014, 2, 1213–1220. [Google Scholar] [CrossRef]
- Rinne, J.W.; Wiltzius, P. Design of holographic structures using genetic algorithms. Opt. Express 2006, 14, 9909–9916. [Google Scholar] [CrossRef] [PubMed]
- Rinne, J.W.; Gupta, S.; Wiltzius, P. Inverse design for phase mask lithography. Opt. Express 2008, 16, 663–670. [Google Scholar] [CrossRef] [PubMed]
- SalehHudin, H.S.; Mohamad, E.N.; Mahadi, W.N.L.; Muhammad Afifi, A. Multiple-jet electrospinning methods for nanofiber processing: A review. Mater. Manuf. Process. 2018, 33, 479–498. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Yu, Q.; Topham, P.D.; Chen, C.; Wang, L. A comprehensive review of electrospinning block copolymers. Soft Matter 2019. [Google Scholar] [CrossRef]
- Khalf, A.; Madihally, S.V. Recent advances in multiaxial electrospinning for drug delivery. Eur. J. Pharm. Biopharm. 2017, 112, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Wang, H.S.; Choe, Y.; Huh, J.; Bang, J. Three-Dimensional Multilayered Nanostructures from Crosslinkable Block Copolymers. ACS Macro Lett. 2016, 5, 287–291. [Google Scholar] [CrossRef]
- Feng, H.; Lu, X.; Wang, W.; Kang, N.-G.; Mays, J.W. Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Guo, H.; Thomas, E.L. Topological defects in tubular network block copolymers. Polymer 2019, 168, 44–52. [Google Scholar] [CrossRef]
- Kwon, Y.W.; Park, J.; Kim, T.; Kang, S.H.; Kim, H.; Shin, J.; Jeon, S.; Hong, S.W. Flexible Near-Field Nanopatterning with Ultrathin, Conformal Phase Masks on Nonplanar Substrates for Biomimetic Hierarchical Photonic Structures. ACS Nano 2016, 10, 4609–4617. [Google Scholar] [CrossRef] [PubMed]
- Kayaci, F.; Vempati, S.; Ozgit-Akgun, C.; Biyikli, N.; Uyar, T. Enhanced photocatalytic activity of homoassembled ZnO nanostructures on electrospun polymeric nanofibers: A combination of atomic layer deposition and hydrothermal growth. Appl. Catal. B Environ. 2014, 156–157, 173–183. [Google Scholar] [CrossRef]
- Biener, M.M.; Ye, J.; Baumann, T.F.; Wang, Y.M.; Shin, S.J.; Biener, J.; Hamza, A.V. Ultra-strong and Low-Density Nanotubular Bulk Materials with Tunable Feature Sizes. Adv. Mater. 2014, 26, 4808–4813. [Google Scholar] [CrossRef] [PubMed]
- Kayaci, F.; Ozgit-Akgun, C.; Donmez, I.; Biyikli, N.; Uyar, T. Polymer–Inorganic Core–Shell Nanofibers by Electrospinning and Atomic Layer Deposition: Flexible Nylon–ZnO Core–Shell Nanofiber Mats and Their Photocatalytic Activity. ACS Appl. Mater. Interfaces 2012, 4, 6185–6194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elam, J.W.; Routkevitch, D.; Mardilovich, P.P.; George, S.M. Conformal Coating on Ultrahigh-Aspect-Ratio Nanopores of Anodic Alumina by Atomic Layer Deposition. Chem. Mater. 2003, 15, 3507–3517. [Google Scholar] [CrossRef]
- Wilson, C.A.; Grubbs, R.K.; George, S.M. Nucleation and Growth during Al2O3 Atomic Layer Deposition on Polymers. Chem. Mater. 2005, 17, 5625–5634. [Google Scholar] [CrossRef]
- Guo, H.C.; Ye, E.; Li, Z.; Han, M.-Y.; Loh, X.J. Recent progress of atomic layer deposition on polymeric materials. Mater. Sci. Eng. C 2017, 70, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Parsons, G.N.; Atanasov, S.E.; Dandley, E.C.; Devine, C.K.; Gong, B.; Jur, J.S.; Lee, K.; Oldham, C.J.; Peng, Q.; Spagnola, J.C.; et al. Mechanisms and reactions during atomic layer deposition on polymers. Coord. Chem. Rev. 2013, 257, 3323–3331. [Google Scholar] [CrossRef]
- Brozena, A.H.; Oldham, C.J.; Parsons, G.N. Atomic layer deposition on polymer fibers and fabrics for multifunctional and electronic textiles. J. Vac. Sci. Technol. A 2016, 34, 010801. [Google Scholar] [CrossRef]
- Haider, A.; Yilmaz, M.; Deminskyi, P.; Eren, H.; Biyikli, N. Nanoscale selective area atomic layer deposition of TiO2 using e-beam patterned polymers. RSC Adv. 2016, 6, 106109–106119. [Google Scholar] [CrossRef]
- Sinha, A.; Hess, D.W.; Henderson, C.L. Area selective atomic layer deposition of titanium dioxide: Effect of precursor chemistry. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2006, 24, 2523–2532. [Google Scholar] [CrossRef]
- Färm, E.; Kemell, M.; Ritala, M.; Leskelä, M. Selective-Area Atomic Layer Deposition Using Poly(methyl methacrylate) Films as Mask Layers. J. Phys. Chem. C 2008, 112, 15791–15795. [Google Scholar] [CrossRef]
- Chen, R.; Bent, S.F. Chemistry for Positive Pattern Transfer Using Area-Selective Atomic Layer Deposition. Adv. Mater. 2006, 18, 1086–1090. [Google Scholar] [CrossRef]
- Seo, S.; Yeo, B.C.; Han, S.S.; Yoon, C.M.; Yang, J.Y.; Yoon, J.; Yoo, C.; Kim, H.-J.; Lee, Y.-B.; Lee, S.J.; et al. Reaction Mechanism of Area-Selective Atomic Layer Deposition for Al2O3 Nanopatterns. ACS Appl. Mater. Interfaces 2017, 9, 41607–41617. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.Z.; Losego, M.D. Vapor phase infiltration (VPI) for transforming polymers into organic–inorganic hybrid materials: A critical review of current progress and future challenges. Mater. Horiz. 2017, 4, 747–771. [Google Scholar] [CrossRef]
- Lee, S.-M.; Pippel, E.; Gösele, U.; Dresbach, C.; Qin, Y.; Chandran, C.V.; Bräuniger, T.; Hause, G.; Knez, M. Greatly Increased Toughness of Infiltrated Spider Silk. Science 2009, 324, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Khan, S.; Rojas, O.J.; Parsons, G.N. Control of Micro- and Mesopores in Carbon Nanofibers and Hollow Carbon Nanofibers Derived from Cellulose Diacetate via Vapor Phase Infiltration of Diethyl Zinc. ACS Sustain. Chem. Eng. 2018, 6, 13844–13853. [Google Scholar] [CrossRef]
- Mackus, A.J.M.; Garcia-Alonso, D.; Knoops, H.C.M.; Bol, A.A.; Kessels, W.M.M. Room-Temperature Atomic Layer Deposition of Platinum. Chem. Mater. 2013, 25, 1769–1774. [Google Scholar] [CrossRef]
- Ozgit-Akgun, C.; Kayaci, F.; Donmez, I.; Uyar, T.; Biyikli, N. Template-Based Synthesis of Aluminum Nitride Hollow Nanofibers Via Plasma-Enhanced Atomic Layer Deposition. J. Am. Ceram. Soc. 2013, 96, 916–922. [Google Scholar] [CrossRef]
- Ozgit-Akgun, C.; Kayaci, F.; Vempati, S.; Haider, A.; Celebioglu, A.; Goldenberg, E.; Kizir, S.; Uyar, T.; Biyikli, N. Fabrication of flexible polymer–GaN core–shell nanofibers by the combination of electrospinning and hollow cathode plasma-assisted atomic layer deposition. J. Mater. Chem. C 2015, 3, 5199–5206. [Google Scholar] [CrossRef] [Green Version]
- Aaltonen, T.; Ritala, M.; Sajavaara, T.; Keinonen, J.; Leskelä, M. Atomic Layer Deposition of Platinum Thin Films. Chem. Mater. 2003, 15, 1924–1928. [Google Scholar] [CrossRef]
- Musschoot, J.; Dendooven, J.; Deduytsche, D.; Haemers, J.; Buyle, G.; Detavernier, C. Conformality of thermal and plasma enhanced atomic layer deposition on a non-woven fibrous substrate. Surf. Coat. Technol. 2012, 206, 4511–4517. [Google Scholar] [CrossRef]
- Ozgit-Akgun, C.; Goldenberg, E.; Bolat, S.; Tekcan, B.; Kayaci, F.; Uyar, T.; Okyay, A.K.; Biyikli, N. Low-temperature hollow cathode plasma-assisted atomic layer deposition of crystalline III-nitride thin films and nanostructures. Phys. Status Solidi C 2015, 12, 394–398. [Google Scholar] [CrossRef] [Green Version]
- Knoops, H.C.M.; Faraz, T.; Arts, K.; Kessels, W.M.M. Status and prospects of plasma-assisted atomic layer deposition. J. Vac. Sci. Technol. A 2019, 37, 030902. [Google Scholar] [CrossRef]
- Sweet, W.J.; Oldham, C.J.; Parsons, G.N. Atomic Layer Deposition of Metal Oxide Patterns on Nonwoven Fiber Mats using Localized Physical Compression. ACS Appl. Mater. Interfaces 2014, 6, 9280–9289. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Park, J.; Jeon, S.G.; Kim, K.; Park, S.H.; Shin, H.S.; Kim, B.; Jeon, S.; Song, J.Y. Monolithic Bi1.5Sb0.5Te3 ternary alloys with a periodic 3D nanostructure for enhancing thermoelectric performance. J. Mater. Chem. C 2017, 5, 8974–8980. [Google Scholar] [CrossRef]
- Li, J.-F.; Liu, W.-S.; Zhao, L.-D.; Zhou, M. High-performance nanostructured thermoelectric materials. NPG Asia Mater. 2010, 2, 152. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, K.H.; Mun, H.A.; Kim, H.S.; Hwang, S.W.; Roh, J.W.; Yang, D.J.; Shin, W.H.; Li, X.S.; Lee, Y.H.; et al. Dense dislocation arrays embedded in grain boundaries for high-performance bulk thermoelectrics. Science 2015, 348, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Minnich, A.J.; Dresselhaus, M.S.; Ren, Z.F.; Chen, G. Bulk nanostructured thermoelectric materials: Current research and future prospects. Energy Environ. Sci. 2009, 2, 466–479. [Google Scholar] [CrossRef]
- Shin, H.S.; Jeon, S.G.; Yu, J.; Kim, Y.-S.; Park, H.M.; Song, J.Y. Twin-driven thermoelectric figure-of-merit enhancement of Bi2Te3 nanowires. Nanoscale 2014, 6, 6158–6165. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; We, J.H.; Cho, B.J. A wearable thermoelectric generator fabricated on a glass fabric. Energy Environ. Sci. 2014, 7, 1959–1965. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, M.-S.; Lee, S.; Kim, C.; Kim, Y.-J. Wearable thermoelectric generator for harvesting human body heat energy. Smart Mater. Struct. 2014, 23, 105002. [Google Scholar] [CrossRef]
- Bahk, J.-H.; Fang, H.; Yazawa, K.; Shakouri, A. Flexible thermoelectric materials and device optimization for wearable energy harvesting. J. Mater. Chem. C 2015, 3, 10362–10374. [Google Scholar] [CrossRef]
- Poodt, P.; Cameron, D.C.; Dickey, E.; George, S.M.; Kuznetsov, V.; Parsons, G.N.; Roozeboom, F.; Sundaram, G.; Vermeer, A. Spatial atomic layer deposition: A route towards further industrialization of atomic layer deposition. J. Vac. Sci. Technol. A 2012, 30, 010802. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Rojas, D.; Maindron, T.; Esteve, A.; Piallat, F.; Kools, J.C.S.; Decams, J.M. Speeding up the unique assets of atomic layer deposition. Mater. Today Chem. 2019, 12, 96–120. [Google Scholar] [CrossRef]
- Muñoz-Rojas, D.; Nguyen, V.H.; Masse de la Huerta, C.; Jiménez, C.; Bellet, D. Spatial Atomic Layer Deposition; InTech: Rijeka, Croatia, 2019. [Google Scholar]







| ALD Type | Template Preparation Method | Template Structure Regularity | Precursor | Reactant/Plasma Gas | Temperature (°C) | Deposited Materials | Ref. |
|---|---|---|---|---|---|---|---|
| Thermal | Electrospinning | Irregular | DEZ | H2O | 200 | ZnO | [69] |
| Thermal | Electrospinning | Irregular | DEZ | H2O | 200 | ZnO | [71] |
| Thermal | Nonwoven fabric | Irregular | DEZ | H2O | 125–155 | ZnO | [92] |
| Thermal | BCP lithography | Regular | TiCl4 | H2O | 150 | TiO2 | [10] |
| Thermal | BCP lithography | Regular | DEZ | H2O | 50 | ZnO | [9] |
| Thermal | DLW | Regular | DEZ + TMA | H2O | 250 | Al:ZnO | [14] |
| Thermal | DLW | Regular | DEZ + TIP | H2O | 250 | Ti:ZnO | [14] |
| Thermal | MBIL | Regular | TiCl4 | H2O | 100 | TiO2 | [20] |
| Thermal | MBIL | Regular | TMA | H2O | 85 | Al2O3 | [18] |
| Thermal | MBIL | Regular | DEZ | H2O | 100 | ZnO | [33] |
| Thermal | PnP | Regular | TDMAT | H2O | 90 | TiO2 | [22] |
| Thermal | PnP | Regular | TDMAT | NH3 | 350 | TiN | [22] |
| Thermal | PnP | Regular | DEZ | H2O | 150 | ZnO | [27] |
| Plasma-enhanced | Nonwoven fabric | Irregular | TMA | O2 | 75 | Al2O3 | [89] |
| Plasma-enhanced | Electrospinning | Irregular | TMA | NH3 | 200 | AlN | [86] |
| Plasma-enhanced | Electrospinning | Irregular | GaMe3 | N2 / H2 | 200 | GaN | [87] |
| Plasma-enhanced | Woven fabric | Irregular | MeCpPtMe3 | O2 / H2 | RT | Pt | [85] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.; Ahn, C.; Jeon, S.; Park, J. Atomic Layer Deposition of Inorganic Thin Films on 3D Polymer Nanonetworks. Appl. Sci. 2019, 9, 1990. https://doi.org/10.3390/app9101990
Ahn J, Ahn C, Jeon S, Park J. Atomic Layer Deposition of Inorganic Thin Films on 3D Polymer Nanonetworks. Applied Sciences. 2019; 9(10):1990. https://doi.org/10.3390/app9101990
Chicago/Turabian StyleAhn, Jinseong, Changui Ahn, Seokwoo Jeon, and Junyong Park. 2019. "Atomic Layer Deposition of Inorganic Thin Films on 3D Polymer Nanonetworks" Applied Sciences 9, no. 10: 1990. https://doi.org/10.3390/app9101990
APA StyleAhn, J., Ahn, C., Jeon, S., & Park, J. (2019). Atomic Layer Deposition of Inorganic Thin Films on 3D Polymer Nanonetworks. Applied Sciences, 9(10), 1990. https://doi.org/10.3390/app9101990