An IoT-Based Non-Invasive Glucose Level Monitoring System Using Raspberry Pi
Abstract
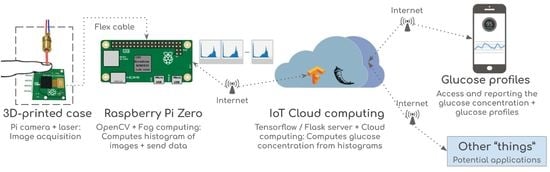
:1. Introduction
2. Proposed Internet of Things (IoT) Glucose Monitoring System
2.1. Materials
2.2. Methods
2.3. Data Acquisition
- The laser-beam is used as the energy source to pass throughout the medium.
- The finger is the medium through which the light will be transmitted.
- The camera acts as the sensor responsible to capture the transmitted light and how it is scattered along the finger.
2.4. Histogram Calculation
2.5. IoT Cloud Computing
2.5.1. Model Selection
- Participants: A total of 514 healthy subjects, with age ranging from 18–44 years, and averaging 28 ± 5.2 years, were invited on a random basis to be part of this study. The training set is constituted by 514 histograms, each representing the average of the 12 histograms acquired for all subjects. Participants of this study were of different skin colour for increasing the variability of the sample, thus making the training data set representative of this region.
- ANN structure: The ANN used in this work is composed of 256 input neurons—the histogram values—and 2 hidden layers, each one composed of 1024 neurons, whereas the output layer has only one neuron corresponding to the glucose concentration value. At the end of both hidden layers, a 0.20 dropout was considered. The activation functions used in this model were ReLU [44] in all cases.
- ANN configuration: The ANN was trained using the ADAM method for error minimisation [45]. A total of 100 epochs with a 50 batch size were also considered.
- Evaluation metrics: With the aim to evaluate the model performance, the mean absolute error and the Clarke error grid analysis [46] were adopted. The mean absolute error (MAE) is computed as in Equation (2) where yi represents the reference glucose values, and stands for the estimated values by the algorithm. In the Clarke error grid, the reference glucose concentrations versus the estimated values are plotted and divided into five zones, as shown in Figure 2. Zones A and B represent accurate or acceptable glucose results, respectively; zone C could lead to unnecessary treatments while zones D and E entail potentially dangerous mistreatment.
- Cross-validation model: The whole input data set was divided into train/test (for model selection) and validation subsets. For this purpose a 10-fold cross-validation scheme was randomly applied to the 70% of the whole data set, representing the train/test subsets; the remaining 30% were taken as the validation subset, as illustrated in Figure 3. In the 10-fold cross-validation, data is randomly divided into 10 groups or folds of approximately equal number of observations. The first fold is used as a test subset whilst the remaining nine folds serve to train the classifier. This procedure is repeated ten times until every fold is treated as a test subset [47].
2.5.2. System Implementation
2.6. Smart End Device
3. Results
3.1. IoT Sensor Setup
3.2. End Device Screens
- Activity 1: login and server connection. This function aims for establishing a connection to the Flask IoT server through the port 5000.
- Activity 2: shows the current glucose value and graphs the last ten measurements for logged user. This is made by JPLOT, a .aar library to graph static (x, y) coordinate points, time series, among other; which is convenient for showing the results in our application.
- Activity 3: show the glucose record profile estimated on previous days. The graph in this screen also displays two lines representing the limits for hypoglucaemia and hyperglucaemia correspondingly enclosing the healthy glucose values.
3.3. Model Selection and Validation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamidi, H. An approach to develop the smart health using Internet of Things and authentication based on biometric technology. Futur. Gener. Comput. Syst. 2019, 91, 434–449. [Google Scholar] [CrossRef]
- Gubbi, J.; Buyya, R.; Marusic, S.; Palaniswami, M. Internet of Things (IoT): A vision, architectural elements, and future directions. Futur. Gener. Comput. Syst. 2013, 29, 1645–1660. [Google Scholar] [CrossRef] [Green Version]
- Borgia, E. The Internet of Things vision: Key features, applications and open issues. Comput. Commun. 2014, 54, 1–31. [Google Scholar] [CrossRef]
- Huang, L.-S.; Su, J.-Y.; Pao, T.-L. A context aware smart classroom architecture for smart campuses. Appl. Sci. 2019, 9, 1837. [Google Scholar] [CrossRef]
- Kim, Y.; Moon, J.; Hwang, E. Constructing differentiated educational materials using semantic annotation for sustainable education in IoT environments. Sustainability 2018, 10, 1296. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.; Jiang, L.; Xie, S.; Zhang, Y. Intelligent edge computing for IoT-Based energy management in smart cities. IEEE Netw. 2019, 33, 111–117. [Google Scholar] [CrossRef]
- Li, S.; Ni, Q.; Sun, Y.; Min, G.; Al-Rubaye, S. Energy-efficient resource allocation for industrial cyber-physical IoT systems in 5G era. IEEE Trans. Ind. Inform. 2018, 14, 2618–2628. [Google Scholar] [CrossRef]
- Garcia-de-Prado, A.; Ortiz, G.; Boubeta-Puig, J. COLLECT: COLLaborativE ConText-aware service oriented architecture for intelligent decision-making in the Internet of Things. Expert Syst. Appl. 2017, 85, 231–248. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Lee, K. The Internet of Things (IoT): Applications, investments, and challenges for enterprises. Bus. Horiz. 2015, 58, 431–440. [Google Scholar] [CrossRef]
- Santos, P.M.; Rodrigues, J.G.P.; Cruz, S.B.; Lourenço, T.; d’Orey, P.M.; Luis, Y.; Rocha, C.; Sousa, S.; Crisóstomo, S.; Queirós, C. PortoLivingLab: An IoT-Based Sensing Platform for Smart Cities. IEEE Internet Things J. 2018, 5, 523–532. [Google Scholar] [CrossRef]
- Plageras, A.P.; Psannis, K.E.; Stergiou, C.; Wang, H.; Gupta, B.B. Efficient IoT-based sensor BIG Data collection—Processing and analysis in smart buildings. Futur. Gener. Comput. Syst. 2018, 82, 349–357. [Google Scholar] [CrossRef]
- Rathore, M.M.; Paul, A.; Hong, W.-H.; Seo, H.; Awan, I.; Saeed, S. Exploiting IoT and big data analytics: Defining Smart Digital City using real-time urban data. Sustain. Cities Soc. 2018, 40, 600–610. [Google Scholar] [CrossRef]
- Lai, X.; Yang, T.; Wang, Z.; Chen, P. IoT Implementation of kalman filter to improve accuracy of air quality monitoring and prediction. Appl. Sci. 2019, 9, 1831. [Google Scholar] [CrossRef]
- Benammar, M.; Abdaoui, A.; Ahmad, S.; Touati, F.; Kadri, A. A modular IoT platform for real-time indoor air quality monitoring. Sensors 2018, 18, 581. [Google Scholar] [CrossRef]
- Dhingra, S.; Madda, R.B.; Gandomi, A.H.; Patan, R.; Daneshmand, M. Internet of things mobile-air pollution monitoring system (IoT-Mobair). IEEE Int. Things J. 2019, 6, 5577–5584. [Google Scholar] [CrossRef]
- De l’Aulnoit, A.H.; Boudet, S.; Génin, M.; Gautier, P.-F.; Schiro, J.; de l’Aulnoit, D.H.; Beuscart, R. Development of a smart mobile data module for fetal monitoring in E-Healthcare. J. Med. Syst. 2018, 42, 83. [Google Scholar] [CrossRef]
- Abdellatif, A.A.; Emam, A.; Chiasserini, C.-F.; Mohamed, A.; Jaoua, A.; Ward, R. Edge-based compression and classification for smart healthcare systems: Concept, implementation and evaluation. Expert Syst. Appl. 2019, 117, 1–14. [Google Scholar] [CrossRef]
- Ferlie, E.B.; Shortell, S.M. Improving the quality of health care in the United Kingdom and the United States: A framework for change. Milbank Q. 2001, 79, 281–315. [Google Scholar] [CrossRef]
- Shen, H.; Ma, D.; Zhao, Y.; Sun, H.; Sun, S.; Ye, R.; Huang, L.; Lang, B.; Sun, Y. MIAPS: A web-based system for remotely accessing and presenting medical images. Comput. Methods Progr. Biomed. 2014, 113, 266–283. [Google Scholar] [CrossRef]
- Segura-Garcia, J.; Garcia-Pineda, M.; Tamarit-Tronch, M.; Cibrian, R.; Salvador-Palmer, R. Cost-Effective eHealth system based on a Multi-Sensor System-on-Chip platform and data fusion in cloud for sport activity monitoring. Electronics 2018, 7, 183. [Google Scholar] [CrossRef]
- Rohokale, V.M.; Prasad, N.R.; Prasad, R. A cooperative Internet of Things (IoT) for rural healthcare monitoring and control. In Proceedings of the 2011 2nd International Conference on Wireless Communication, Vehicular Technology, Information Theory and Aerospace & Electronic Systems Technology (Wireless VITAE), Chennai, India, 28 February–3 March 2011; IEEE: New York, NY, USA, 2011; pp. 1–6. [Google Scholar]
- Doukas, C.; Maglogiannis, I. Bringing IoT and cloud computing towards pervasive healthcare. In Proceedings of the 2012 Sixth International Conference on Innovative Mobile and Internet Services in Ubiquitous Computing, Palermo, Italy, 4–6 July 2012; IEEE: New York, NY, USA, 2012; pp. 922–926. [Google Scholar]
- WHO: World Health Organization Diabetes Factsheets. Available online: https://www.who.int/en/news-room/fact-sheets/detail/diabetes (accessed on 27 July 2019).
- Guariguata, L.; Whiting, D.; Weil, C.; Unwin, N. The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res. Clin. Pract. 2011, 94, 322–332. [Google Scholar] [CrossRef]
- Lin, T.; Gal, A.; Mayzel, Y.; Horman, K.; Bahartan, K. Non-invasive glucose monitoring: A review of challenges and recent advances. Curr. Trends Biomed. Eng. Biosci. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Talib, A.J.; Alkahtani, M.; Jiang, L.; Alghannam, F.; Brick, R.; Gomes, C.L.; Scully, M.O.; Sokolov, A.V.; Hemmer, P.R. Lanthanide ions doped in vanadium oxide for sensitive optical glucose detection. Opt. Mater. Express 2018, 8, 3277–3287. [Google Scholar] [CrossRef] [Green Version]
- Blum, Z.; Pankratov, D.; Shleev, S. Powering electronic contact lenses: Current achievements, challenges, and perspectives. Expert Rev. Ophthalmol. 2014, 9, 269–273. [Google Scholar] [CrossRef]
- Do Amaral, C.E.F.; Wolf, B. Current development in non-invasive glucose monitoring. Med. Eng. Phys. 2008, 30, 541–549. [Google Scholar] [CrossRef]
- Vashist, S.K. Non-invasive glucose monitoring technology in diabetes management: A review. Anal. Chim. Acta 2012, 750, 16–27. [Google Scholar] [CrossRef]
- So, C.-F.; Choi, K.-S.; Wong, T.K.S.; Chung, J.W.Y. Recent advances in noninvasive glucose monitoring. Med. Devices (Auckl. NZ) 2012, 5, 45. [Google Scholar]
- Yadav, J.; Rani, A.; Singh, V.; Murari, B.M. Prospects and limitations of non-invasive blood glucose monitoring using near-infrared spectroscopy. Biomed. Signal Process. Control 2015, 18, 214–227. [Google Scholar] [CrossRef]
- Waynant, R.W.; Ilev, I.K.; Gannot, I. Mid-Infrared laser applications in medicine and biology. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2001, 359, 635–644. [Google Scholar] [CrossRef]
- Tura, A.; Maran, A.; Pacini, G. Non-invasive glucose monitoring: Assessment of technologies and devices according to quantitative criteria. Diabetes Res. Clin. Pract. 2007, 77, 16–40. [Google Scholar] [CrossRef]
- Jeon, K.J.; Hwang, I.D.; Hahn, S.J.; Yoon, G. Comparison between transmittance and reflectance measurements in glucose determination using near infrared spectroscopy. J. Biomed. Opt. 2006, 11, 14022. [Google Scholar] [CrossRef]
- Chen, T.-L.; Lo, Y.-L.; Liao, C.-C.; Phan, Q.-H. Noninvasive measurement of glucose concentration on human fingertip by optical coherence tomography. J. Biomed. Opt. 2018, 23, 47001. [Google Scholar] [CrossRef]
- Shao, J.; Lin, M.; Li, Y.; Li, X.; Liu, J.; Liang, J.; Yao, H. In vivo blood glucose quantification using Raman spectroscopy. PLoS ONE 2012, 7, e48127. [Google Scholar] [CrossRef]
- Ali, H.; Bensaali, F.; Jaber, F. Novel approach to non-invasive blood glucose monitoring based on transmittance and refraction of visible laser light. IEEE Access 2017, 5, 9163–9174. [Google Scholar] [CrossRef]
- Kocsis, L.; Herman, P.; Eke, A. The modified Beer—Lambert law revisited. Phys. Med. Biol. 2006, 51, N91. [Google Scholar] [CrossRef]
- Francisco-García, V.; Guzmán-Guzmán, I.P.; Salgado-Rivera, R.; Alonso-Silverio, G.A.; Alarcón-Paredes, A. Non-invasive Glucose Level Estimation: A Comparison of Regression Models Using the MFCC as Feature Extractor. In Proceedings of the Mexican Conference on Pattern Recognition, Querétaro, México, 18 May 2019; Springer: Berlin, Germany, 2019; pp. 206–215. [Google Scholar]
- Abadi, M.; Agarwal, A.; Barham, P.; Brevdo, E.; Chen, Z.; Citro, C.; Corrado, G.S.; Davis, A.; Dean, J.; Devin, M. Tensorflow: Large-scale machine learning on heterogeneous distributed systems. arXiv 2016, arXiv:1603.04467. [Google Scholar]
- Pallets Projects Flask. Available online: https://flask.palletsprojects.com (accessed on 16 July 2019).
- Alarcón-Paredes, A.; Rebolledo-Nandi, Z.; Guzmán-Guzmán, I.P.; Yáñez-Márquez, C.; Alonso, G.A. A non-invasive glucose level estimation in a multi-sensing health care monitoring system. Technol. Heal. Care 2018, 26, 203–208. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 50–60. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016; ISBN 0262337371. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Clarke, W.L. The original Clarke error grid analysis (EGA). Diabetes Technol. Ther. 2005, 7, 776–779. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: Berlin, Germany, 2013; Volume 6. [Google Scholar]
- Monte-Moreno, E. Non-invasive estimate of blood glucose and blood pressure from a photoplethysmograph by means of machine learning techniques. Artif. Intell. Med. 2011, 53, 127–138. [Google Scholar] [CrossRef]
- Segman, Y. Device and method for noninvasive glucose assessment. J. Diabetes Sci. Technol. 2018, 12, 1159–1168. [Google Scholar] [CrossRef]
- Harman-Boehm, I.; Gal, A.; Raykhman, A.M.; Naidis, E.; Mayzel, Y. Noninvasive glucose monitoring: Increasing accuracy by combination of multi-technology and multi-sensors. J. Diabetes Sci. Technol. 2010, 4, 583–595. [Google Scholar] [CrossRef]








| Endpoint | Method | Activity |
|---|---|---|
| /signUp | POST | Creates a new user |
| /glucose/estimation | POST | Requests the histograms and estimates the glucose level |
| /glucose/query | GET | Requests a query to the database and delivers the ten most up-to-date values |
| Train/Test Subset | Validation Subset | |
|---|---|---|
| MAE | 11.81 | 10.37 |
| Clarke grid error zone (%) (A-B-C-D-E) | (83.84-16.16-0-0-0) | (90.32-9.68-0-0-0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarcón-Paredes, A.; Francisco-García, V.; Guzmán-Guzmán, I.P.; Cantillo-Negrete, J.; Cuevas-Valencia, R.E.; Alonso-Silverio, G.A. An IoT-Based Non-Invasive Glucose Level Monitoring System Using Raspberry Pi. Appl. Sci. 2019, 9, 3046. https://doi.org/10.3390/app9153046
Alarcón-Paredes A, Francisco-García V, Guzmán-Guzmán IP, Cantillo-Negrete J, Cuevas-Valencia RE, Alonso-Silverio GA. An IoT-Based Non-Invasive Glucose Level Monitoring System Using Raspberry Pi. Applied Sciences. 2019; 9(15):3046. https://doi.org/10.3390/app9153046
Chicago/Turabian StyleAlarcón-Paredes, Antonio, Victor Francisco-García, Iris P. Guzmán-Guzmán, Jessica Cantillo-Negrete, René E. Cuevas-Valencia, and Gustavo A. Alonso-Silverio. 2019. "An IoT-Based Non-Invasive Glucose Level Monitoring System Using Raspberry Pi" Applied Sciences 9, no. 15: 3046. https://doi.org/10.3390/app9153046
APA StyleAlarcón-Paredes, A., Francisco-García, V., Guzmán-Guzmán, I. P., Cantillo-Negrete, J., Cuevas-Valencia, R. E., & Alonso-Silverio, G. A. (2019). An IoT-Based Non-Invasive Glucose Level Monitoring System Using Raspberry Pi. Applied Sciences, 9(15), 3046. https://doi.org/10.3390/app9153046