Immunoexpression of Macroh2a in Uveal Melanoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of UMs
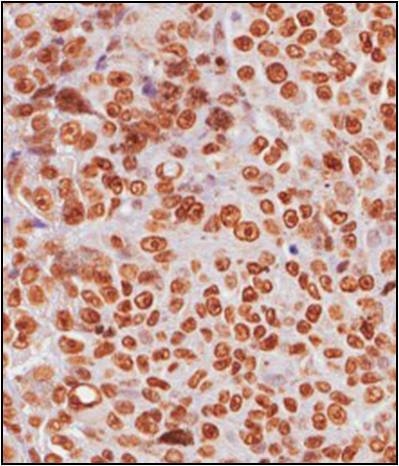
3.2. mH2A.1 Expression and Clinicopathological Features in UMs
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pukrushpan, P.; Tulvatana, W.; Pittayapongpat, R. Congenitaluvealmalignantmelanoma. J. AAPOS 2014, 18, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, F.; Caltabiano, G.; Queirolo, P. Uveal melanoma. Cancer Treat. Rev. 2012, 38, 549–553. [Google Scholar] [CrossRef] [PubMed]
- McLean, I.W.; Foster, W.D.; Zimmerman, L.E.; Gamel, J.W. Modifications of Callender’s classi fication of uveal melanoma at the Armed Forces Institute of Pathology. Am. J. Ophthalmol. 1983, 96, 502–509. [Google Scholar] [CrossRef]
- Willson, J.K.; Albert, D.M.; Moy, C.S. Collaborative Ocular Melanoma Study Group, Assessment of metastaticdisease status atdeath in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch. Ophthalmol. 2001, 119, 670–676. [Google Scholar] [CrossRef]
- Cantariño, N.; Douet, J.; Buschbeck, M. MacroH2A–An epigeneticregulator of cancer. Cancer Lett. 2013, 336, 247–252. [Google Scholar] [CrossRef]
- Kapoor, A.; Goldberg, M.S.; Cumberland, L.K.; Ratnakumar, K.; Segura, M.F.; Emanuel, P.O.; Menendez, S.; Vardabasso, C.; Leroy, G.; Vidal, C.I.; et al. The histonevariant macroH2A suppresses melanoma progressionthroughregulation of CDK8. Nature 2010, 468, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Long, J.; Li, J. MacroH2A suppresses the proliferation of the B16 melanoma cell line. Mol. Med. Rep. 2014, 10, 1845–1850. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, M.M.; Triozzi, P.L.; Singh, A.D. Uveal melanoma: Evidence for adjuvanttherapy. Int. Ophthalmol. Clin. 2015, 55, 45–51. [Google Scholar] [CrossRef]
- Caltabiano, R.; Puzzo, L.; Barresi, V.; Ieni, A.; Loreto, C.; Musumeci, G.; Castrogiovanni, P.; Ragusa, M.; Foti, P.; Russo, A.; et al. ADAM 10 expression in primaryuveal melanoma asprognosticfactor for risk of metastasis. Pathol. Res. Pract. 2016, 212, 980–987. [Google Scholar] [CrossRef]
- Augsburger, J.J.; Corrêa, Z.M.; Trichopoulos, N. Surveillancetesting for metastasis from primaryuveal melanoma and effect on patientsurvival. Am. J. Ophthalmol. 2011, 152, 5–9. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare butdeadlycancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Caltabiano, R.; Puzzo, L.; Barresi, V.; Cardile, V.; Loreto, C.; Ragusa, M.; Russo, A.; Reibaldi, M.; Longo, A. Expression of RafKinaseInhibitorProtein (RKIP) is apredictor of uveal melanoma Metastasis. Histol Histopathol. 2014, 29, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Salvatorelli, L.; Puzzo, L.; Russo, A.; Reibaldi, M.; Longo, A.; Ragusa, M.; Aldo, C.; Rappazzo, G.; Caltabiano, R.; Salemi, M. Immunoexpression of SPANX-C in metastaticuveal melanoma. Pathol. Res. Pract. 2019, 29, 152431. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.L.; Helms, C.; Bowcock, A.M.; et al. Frequentmutation of BAP1 in metastasizinguvealmelanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, J.C.; de Ayala Alonso, A.G.; Oktaba, K.; Ly-Hartig, N.; McGinty, R.K.; Fraterman, S. Histone H2A deubiquitinaseactivity of the Polycomb repressive complex PR-DUB. Nature 2010, 465, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Machida, Y.J.; Machida, Y.; Vashisht, A.A.; Wohlschlegel, J.A.; Dutta, A. The deubiquitinatingenzyme BAP1 regulatescellgrowth via interaction with HCF-1. J. Biol. Chem. 2009, 284, 34179–34188. [Google Scholar] [CrossRef] [PubMed]
- Costanzi, C.; Pehrson, J.R. Histone macroH2A1 isconcentrated in the inactive X chromosome offemalemammals. Nature 1998, 393, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Poustovoitov, M.V.; Ye, X.; Santos, H.A.; Chen, W.; Daganzo, S.M.; Erzberger, J.P.; Dunbrack, R.L.; Adams, P.D.; Berger, J.M.; et al. Formation ofMacroH2A-containing senescence-associatedheterochromatin foci and senescencedriven by ASF1aand HIRA. Dev. Cell. 2005, 8, 19–30. [Google Scholar] [CrossRef]
- Bernstein, E.; Muratore-Schroeder, T.L.; Diaz, R.L.; Chow, J.C.; Changolkar, L.N.; Shabanowitz, J.; Heard, E.; Pehrson, J.R.; Hunt, D.F.; Allis, C.D. A phosphorylated subpopulation of the histone variant macroH2A1 is excluded from the inactive X chromosome and enriched during mitosis. Proc. Natl. Acad. Sci. USA 2008, 105, 1533–1538. [Google Scholar] [CrossRef] [Green Version]
- Lo Re, O.; Fusilli, C.; Rappa, F.; Van Haele, M.; Douet, J.; Pindjakova, J.; Pata, L.; Vinciguerra, M.; Mazza, T.; Buschbeck, M.; et al. Induction of cancercellstemness by depletion of macrohistone H2A1 in hepatocellular carcinoma. Hepatology 2018, 67, 636–650. [Google Scholar] [CrossRef]
- Hua, S.; Kallen, C.B.; Dhar, R.; Baquero, M.T.; Mason, C.E.; Russell, B.A.; White, K.P.; Rimm, D.L.; Krausz, T.N.; Shah, P.K.; et al. Genomicanalysis of estrogencascaderevealshistonevariant H2A.Z associated with breastcancerprogression. Mol. Syst. Biol. 2008, 4, 188. [Google Scholar] [CrossRef] [PubMed]




| Sex | Age (years) | Location | Thickness (mm) | Largest Diameter (mm) | Cell Type | Pathological T Stage | DFS (Months) | Follow-Up (Months) | MacroH2A | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IS | ES | IRS | ||||||||||
| F | 29 | Ch | 14.2 | 16.2 | Mc | pT2a | 138 | 138 | 3 | 4 | 12 | H |
| F | 83 | Ch/CB | 14.84 | 16.8 | Mc | pT2b | 123 | 123 | 0 | 0 | 0 | L |
| F | 55 | Ch | 9.8 | 13.9 | Sc | pT2a | 122 | 122 | 0 | 0 | 0 | L |
| F | 30 | Ch/CB | 12.05 | 9.2 | Sc | pT2b | 122 | 122 | 2 | 1 | 2 | L |
| M | 74 | Ch/CB | 10.04 | 16.1 | Sc | pT2b | 121 | 121 | 3 | 4 | 12 | H |
| M | 64 | Ch | 7.7 | 11.5 | Sc | pT1a | 112 | 112 | 3 | 4 | 12 | H |
| F | 36 | Ch | 5.81 | 12.7 | Sc | pT1a | 109 | 109 | 3 | 1 | 3 | L |
| F | 59 | Ch | 8.4 | 16.7 | Mc | pT2a | 108 | 108 | 2 | 2 | 4 | L |
| M | 36 | Ch | 6.47 | 9.8 | Mc | pT1a | 108 | 108 | 0 | 0 | 0 | L |
| M | 84 | Ch/CB | 11.9 | 14.8 | Mc | pT2b | 106 | 106 | 0 | 0 | 0 | L |
| F | 67 | Ch | 10.42 | 13.02 | Mc | pT3a | 105 | 105 | 0 | 0 | 0 | L |
| M | 73 | Ch | 9.7 | 11.3 | Mc | pT2a | 102 | 102 | 0 | 0 | 0 | L |
| F | 45 | Ch | 13.7 | 10.2 | Mc | pT2a | 96 | 96 | 0 | 0 | 0 | L |
| M | 58 | Ch | 13.1 | 14.3 | Mc | pT2a | 96 | 96 | 2 | 1 | 2 | L |
| M | 63 | Ch | 3.3 | 11.7 | Sc | pT2a | 85 | 85 | 2 | 3 | 6 | L |
| M | 54 | Ch | 6.32 | 10 | Sc | pT2a | 83 | 83 | 3 | 4 | 12 | H |
| F | 84 | Ch | 11.7 | 17.4 | Mc | pT3a | 78 | 78 | 3 | 4 | 12 | H |
| M | 73 | Ch | 9.24 | 17.7 | Ec | pT2a | 72 | 72 | 2 | 1 | 2 | L |
| M | 83 | Ch | 10.62 | 9.4 | Ec | pT3a | 72 | 72 | 3 | 4 | 12 | H |
| F | 71 | Ch | 3.68 | 6.4 | Ec | pT1a | 71 | 71 | 0 | 0 | 0 | L |
| M | 55 | Ch/CB | 7.5 | 8.9 | Ec | pT2b | 61 | 61 | 3 | 4 | 12 | H |
| M | 52 | Ch | 9.2 | 12.1 | Sc | pT2b | 60 | 60 | 3 | 4 | 12 | H |
| M | 46 | Ch | 8.76 | 11.3 | Sc | pT2a | 54 | 54 | 0 | 0 | 0 | L |
| F | 76 | Ch | 8.02 | 10.7 | Mc | pT1a | 48 | 48 | 3 | 2 | 6 | L |
| F | 63 | Ch | 10.3 | 13.7 | Mc | pT2a | 42 | 42 | 0 | 0 | 0 | L |
| F | 41 | Ch | 5.85 | 10.3 | Mc | pT1a | 42 | 42 | 0 | 0 | 0 | L |
| F | 55 | Ch | 3.2 | 7.6 | Mc | pT2a | 24 | 24 | 2 | 4 | 8 | L |
| F | 74 | Ch | 8.6 | 10.2 | Mc | pT4b | 24 | 24 | 2 | 1 | 2 | L |
| M | 68 | Ch/CB | 10.1 | 10.1 | Ec | pT1b | 24 | 24 | 0 | 0 | 0 | L |
| M | 74 | Ch/CB | 14.45 | 17.5 | Ec | pT4b | 18 | 18 | 2 | 1 | 2 | L |
| M | 70 | Ch/CB | 16.27 | 20.8 | Sc | pT4b | 12 | 12 | 0 | 0 | 0 | L |
| M | 66 | Ch | 9.2 | 14.1 | Mc | pT3a | 12 | 12 | 1 | 1 | 1 | L |
| Sex | Age (years) | Location | Thickness (mm) | Largest Diameter (mm) | Cell Type | Pathological T Stage | DFS (Months) | Follow-Up (Months) | MacroH2A | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IS | ES | IRS | ||||||||||
| F | 58 | Ch | 6.04 | 17.8 | Mc | pT2a | 63 | 64 (†) | 3 | 3 | 9 | L |
| M | 69 | Ch | 7.21 | 15.8 | Mc | pT2a | 54 | 81 (†) | 3 | 4 | 12 | H |
| F | 75 | Ch/CB | 15.5 | 15.3 | Mc | pT3b | 44 | 62 (†) | 3 | 4 | 12 | H |
| F | 50 | Ch | 7.36 | 15.6 | Ec | pT2a | 41 | 81 | 3 | 4 | 12 | H |
| M | 62 | Ch | 13.68 | 16 | Mc | pT3a | 38 | 51 (†) | 3 | 4 | 12 | H |
| F | 51 | Ch/CB | 11.4 | 18.5 | Mc | pT3b | 38 | 61 | 3 | 4 | 12 | H |
| M | 71 | Ch | 13.14 | 17.1 | Ec | pT3a | 33 | 34 (†) | 3 | 4 | 12 | H |
| M | 76 | Ch/CB | 11.6 | 6.5 | Mc | pT1a | 31 | 39 | 0 | 0 | 0 | L |
| M | 72 | Ch | 10.3 | 15.4 | Mc | pT3b | 27 | 35 (†) | 3 | 4 | 12 | H |
| F | 85 | Ch/CB | 7.3 | 14.7 | Sc | pT2d (EE) | 26 | 49 (†) | 3 | 4 | 12 | H |
| M | 73 | Ch | 5.73 | 11.7 | Ec | pT2a | 26 | 42 (†) | 3 | 4 | 12 | H |
| F | 51 | Ch | 9.42 | 19 | Mc | pT3a | 25 | 39 | 1 | 1 | 1 | L |
| F | 74 | Ch | 5.7 | 12.1 | Sc | pT2a | 24 | 37 (†) | 3 | 4 | 12 | H |
| F | 67 | Ch | 3.49 | 20 | Mc | pT4a | 24 | 31 (†) | 3 | 4 | 12 | H |
| M | 74 | Ch | 11.35 | 10.5 | Ec | pT3a | 19 | 47 | 3 | 4 | 12 | H |
| M | 82 | Ch | 9.7 | 11 | Ec | pT2a | 19 | 42 | 3 | 4 | 12 | H |
| F | 72 | Ch | 6.7 | 15.2 | Ec | pT2a | 14 | 28 (†) | 3 | 4 | 12 | H |
| M | 76 | Ch | 13.7 | 17.1 | Mc | pT2a | 14 | 70 | 3 | 4 | 12 | H |
| M | 79 | Ch | 13.91 | 16.1 | Ec | pT3b | 13 | 38 | 3 | 4 | 12 | H |
| F | 66 | Ch/CB | 8.95 | 12.5 | Mc | pT2b | 12 | 37 (†) | 3 | 4 | 12 | H |
| F | 60 | Ch | 8.25 | 16.5 | Ec | pT2a | 11 | 37 (†) | 3 | 4 | 12 | H |
| F | 57 | Ch/CB | 13.6 | 19 | Ec | pT2b | 6 | 55 | 3 | 4 | 12 | H |
| M | 72 | Ch/CB | 13.3 | 15.4 | Mc | pT3b | 0 | 51 | 3 | 4 | 12 | H |
| Sex | Age | Location | Thickness | Largest Diameter | Cell Type | Extrascleral | Pathological | DFS (Months) | Follow-Up (Months) | Macro H2A | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| m–f | (Years) | Extension | T Stage | ||||||||
| All | 28–27 | 67 | Ch 40 | 9.7 | 14.1 | Ec: 15 | No: 54 | pT1a: 7 | 42 | 60 | 12 |
| (n = 55) | (29–85) | Ch/CB 15 | (3.2–16.3) | (6.4–20.8) | Sc: 12 | Yes: 1 | pT1b:1 | (0–138) | (8–138) | (0–12) | |
| Mc: 28 | pT2a: 21 | ||||||||||
| pT2b: 8 | |||||||||||
| pT2d: 1 | |||||||||||
| pT3a: 8 | |||||||||||
| pT3b: 5 | |||||||||||
| pT4a: 1 | |||||||||||
| pT4b: 3 | |||||||||||
| Metastasis-free | 17–15 | 64 | Ch24 | 9.5 | 11.9 | Ec: 6 | No: 32 | pT1a: 6 | 81 | 81 | 2 |
| (n = 32) | (29–84) | Ch/CB 8 | (3.2–16.3) | (6.4–20.8) | Sc: 10 | pT1b: 1 | (12–138) | (8–138) | (0–12) | ||
| Mc: 16 | pT2a: 12 | ||||||||||
| pT2b: 6 | |||||||||||
| pT3a: 4 | |||||||||||
| pT4b: 3 | |||||||||||
| Metastasis | 11–12 | 72 | Ch 16 | 9.7 | 15.6 | Ec: 9 | No: 22 | pT1a: 1 | 25 | 42 | 12 |
| (n = 23) | (50–85) | Ch/CB 7 | (3.5–15.5) | (6.5–20) | Sc: 2 | Yes: 1 | pT2a: 9 | (0–63) | 13 deaths | (0–12) | |
| Mc: 12 | pT2b: 2 | (28–81) | |||||||||
| pT2d: 1 | |||||||||||
| pT3a: 4 | |||||||||||
| pT3b: 5 | |||||||||||
| Pt4b: 1 | |||||||||||
| p (metastasis-free vs metastasis) | 0.400 * | 0.762 ° | 0.911 * | 0.007 * | 0.400 * | 0.418° | 0.560 * | <0.001 * | 0.001 * | <0.001 * | |
| mH2A | Metastasis (n = 23) | Metastasis-Free (n = 32) |
|---|---|---|
| Low | 3 (13%) * | 24 (75%) |
| High | 20 (87%) | 8 (25%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatorelli, L.; Puzzo, L.; Bartoloni, G.; Palmucci, S.; Longo, A.; Russo, A.; Reibaldi, M.; Vinciguerra, M.; Li Volti, G.; Caltabiano, R. Immunoexpression of Macroh2a in Uveal Melanoma. Appl. Sci. 2019, 9, 3244. https://doi.org/10.3390/app9163244
Salvatorelli L, Puzzo L, Bartoloni G, Palmucci S, Longo A, Russo A, Reibaldi M, Vinciguerra M, Li Volti G, Caltabiano R. Immunoexpression of Macroh2a in Uveal Melanoma. Applied Sciences. 2019; 9(16):3244. https://doi.org/10.3390/app9163244
Chicago/Turabian StyleSalvatorelli, Lucia, Lidia Puzzo, Giovanni Bartoloni, Stefano Palmucci, Antonio Longo, Andrea Russo, Michele Reibaldi, Manlio Vinciguerra, Giovanni Li Volti, and Rosario Caltabiano. 2019. "Immunoexpression of Macroh2a in Uveal Melanoma" Applied Sciences 9, no. 16: 3244. https://doi.org/10.3390/app9163244
APA StyleSalvatorelli, L., Puzzo, L., Bartoloni, G., Palmucci, S., Longo, A., Russo, A., Reibaldi, M., Vinciguerra, M., Li Volti, G., & Caltabiano, R. (2019). Immunoexpression of Macroh2a in Uveal Melanoma. Applied Sciences, 9(16), 3244. https://doi.org/10.3390/app9163244