Nucleation Triggering of Highly Undercooled Xylitol Using an Air Lift Reactor for Seasonal Thermal Energy Storage
Abstract
:Featured Application
Abstract
1. Introduction
2. Attempts of Nucleation Triggering and of Discharge at an Acceptable Power
2.1. Materials
2.2. Xylitol-Conscious Nucleation and Crystallization: Commonly Used Techniques
- Local cooling. It fails to trigger nucleation in severely undercooled Xylitol due to the very high activation energy required for atoms diffusion and rearrangement at the solid–liquid interface.
- Intentional seeding. It allows triggering Xylitol crystallization, even in cases with very high undercooled melts. However, the effect of seeding on nucleation is too local. The seed growth being too slow (4 days/10 mL), this technique will lead to very low heat release rates and too long discharge times for the aimed application.
- Ultrasonication. High-power ultrasonification (450 W) allows also crystallizing the studied Xylitol. However, the crystallization rates are still too low for this technique to become appropriate at the storage system scale.
- Solvents addition in Xylitol. It does not contribute to accelerate crystallization even in combination with ultrasonification.
2.3. Successful Xylitol Nucleation and Crystallization: Preliminary Tests
2.3.1. Undercooled Xylitol Crystallization Induced by Mechanical Agitation
2.3.2. Stirring by Bubbling
3. Undercooled Xylitol Crystallization Induced by Bubbling
3.1. Air Lift Reactors
- Reactor n°1. The reactor is a cylindrical glass beaker with a diameter of 85 mm and a height of 105 mm, filled with the molten sugar alcohol (SA) up to 60 mm of height. Air bubbles are generated at the bottom of the vessel by a single tube connected to a pump. The inner diameter of the tube is 3 mm. The pump can provide an air flow rate of 80 L·h−1.
- Reactor n°2. The reactor is a cylindrical glass crystallizer with a diameter of 140 mm diameter and a height of 105 mm, filled with the molten SA up to 60 mm of height. As in reactor n°1, air bubbles are generated at the bottom of the vessel by a single tube (3 mm inner diameter) connected to a pump (80 L·h−1).
3.2. Bubbles Observation into Molten Xylitol
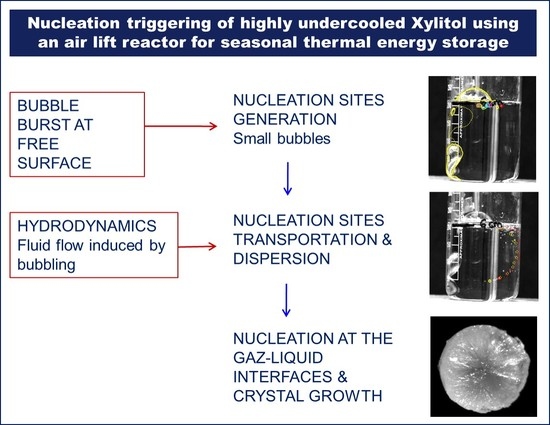
- Bubbles are formed continuously at the submerged orifice. First they grow, detach from the orifice and rise in the melt up to the liquid free-surface. The bubbles generation is periodic and induces an oscillatory motion of the liquid, with a major circulation cell which fills the entire column. Snapshots in Figure 5 illustrates the beat of the free-surface of the liquid.
- At the free-surface of the liquid, the bubbles burst in a very short time and generate smaller bubbles (see Figure 6 (colored circles)). Part of these smaller bubbles are caught by the fluid and dragged by the flow cell as shown in Figure 6. As it can be seen in Figure 7, the small bubbles are then dispersed within the liquid and their amount increases with time.
- After an average stirring time of 5 min of the mixture “bubbles/Xylitol”, Xylitol starts to crystallize. Crystallization begins on the surface of the bubble, at the interface air/Xylitol, then it progresses in the outward direction forming a spherical solid shell.
4. Preliminary Thermal Analysis as a Proof of Feasibility
- The induction period, from the beginning of bubbling to the beginning of crystallization. In a perfectly adiabatic reactor, the temperature of the melt should be constant during this period. In practice, it slightly decreases due to thermal losses. Thermal losses take place both, by conduction through the reactor wall and by convection due to air bubbling at ambient temperature.
- The recalescence period, where Xylitol is crystallizing and its temperature raises. In a perfectly thermally insulated system, Xylitol should reach the melting point and the crystallization process should stop at that moment. However, due to thermal losses, the maximum temperature reached by Xylitol is inferior to its melting point and the crystallization can progress beyond this point. We consider in the following that the recalescence period ends when Xylitol reaches its maximum temperature.
- The end-period, where crystallization (if any) is driven by heat extraction due to thermal losses.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problems formulation for latent heat thermal energy storage systems. Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Duquesne, M.; Toutain, J.; Sempey, A.; Ginestet, S.; del Barrio, E.P. Modeling of a non-linear thermochemical energy storage by adsorption on zeolites. Appl. Therm. Eng. 2014, 71, 469–480. [Google Scholar] [CrossRef]
- Zalba, B.; Marin, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- D’Avignon, K.; Kummert, M. Experimental assessment as a phase change material storage tank. Appl. Therm. Eng. 2016, 99, 880–891. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Garcia, A.; Fernandèz, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Nguyen, P.T.N.; Ilrich, J. Sugar alcohols—Multifunctional agents in the freeze casting process of foods. J. Food Eng. 2015, 153, 1–7. [Google Scholar] [CrossRef]
- Fujii, K.; Izutsu, K.I.; Kume, M.; Yoshino, T.; Yoshihashi, Y.; Sugano, K.; Terada, K. Physical characterization of meso-erythritol as a crystalline bulking agent for freeze-dried formulations. Chem. Pharm. Bull. 2015, 63, 311–317. [Google Scholar] [CrossRef]
- SAM.SSA Project. Sugar Alcohol based Materials for Seasonal Storage Applications. ENER/FP7/296006. 2012–2015. Available online: https://cordis.europa.eu/project/rcn/103643/factsheet/en (accessed on 11 January 2019).
- Solé, A.; Neumann, H.; Niedermaier, S.; Martorell, I.; Schossig, P.; Cabeza, L.F. Stability of sugar alcohols as PCM for thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 126, 125–134. [Google Scholar] [CrossRef]
- Nomura, T.; Zhu, C.; Sagara, A.; Okinaka, N.; Akiyama, T. Estimation of thermal endurance of multicomponent sugar alcohols as phase change materials. Appl. Therm. Eng. 2015, 75, 481–486. [Google Scholar] [CrossRef]
- Ona, E.P.; Zhang, X.; Kyaw, K.; Watanabe, F.; Matsuda, H.; Kakiuchi, H.; Yabe, M.; Chihara, S. Relaxation of Supercooling of Erythritol for Latent Heat Storage. J. Chem. Eng. Jpn. 2001, 34, 376–382. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Pan, R.; Chiu, J.N.; Martin, V. Polyols as phase change materials for surplus thermal energy storage. Appl. Energy 2015, 162, 1439–1452. [Google Scholar] [CrossRef]
- Biçer, A.; Sari, A. Synthesis and thermal energy storage properties of xylitol pentastearate and xylitol pentapalmitate as novel solid-liquid PCMs. Sol. Energy Mater. Sol. Cells 2012, 102, 215–226. [Google Scholar] [CrossRef]
- Höhlein, S.; König-Haagen, A.; Brüggemann, D. Thermophysical characterization of MgCl2.H2O, Xylitol and Erythritol as Phase Change Materials (PCM) for Latent Heat Thermal Energy Storage (LHTES). Materials 2017, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liu, Q.; Zhao, J.; Jui, G.; Wu, W.; Yan, J.; Li, H.; Jin, H. Mobilized thermal nergy storage: Materials, containers and economic evaluation. Energy Convers. Manag. 2018, 177, 315–329. [Google Scholar] [CrossRef]
- Del Barrio, E.P.; Godin, A.; Duquesne, M.; Daranlot, J.; Jolly, J.; Alshaer, W.; Kouadio, T.; Sommier, A. Characterization of different sugar alcohols as phase change materials for thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2017, 159, 560–569. [Google Scholar] [CrossRef]
- Duquesne, M.; Godin, A.; del Barrio, E.P.; Achchaq, F. Crystal growth kinetics of sugar alcohols as phase change materials for thermal energy storage. Energy Procedia 2017, 139, 315–321. [Google Scholar] [CrossRef]
- Godin, A.; Duquesne, M.; del Barrio, E.P.; Achchaq, F.; Monneyron, P. Bubble agitation as a new low intrusive method to crystallize glass-forming materials. Energy Procedia 2017, 139, 352–357. [Google Scholar] [CrossRef]
- Vedantam, S.; Ranade, V. Crystallization: Key thermodynamic, kinetic and hydrodynamic aspects. Sadhana 2013, 38, 1287–1337. [Google Scholar] [CrossRef]
- Gaddis, E.; Vogelpohl, A. Bubble formation in quiescent liquids under constant flow conditions. Chem. Eng. Sci. 1986, 41, 97–105. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Joshi, J.B. Bubble formation and bubble rise velocity in gas-liquid systems: A review. Ind. Eng. Chem. Res. 2005, 44, 5873–5931. [Google Scholar] [CrossRef]
- Abdulmouti, H. Bubbly two-phase flow: Part II—Characteristics and parameters. Am. J. Fluids Dyn. 2014, 4, 115–180. [Google Scholar]
- Rollbusch, P.; Bothe, M.; Becker, M.; Ludwig, M. Bubble columns operated under industrially relevant conditions—Current understanding of design parameters. Chem. Eng. Sci. 2015, 126, 660–678. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duquesne, M.; Palomo Del Barrio, E.; Godin, A. Nucleation Triggering of Highly Undercooled Xylitol Using an Air Lift Reactor for Seasonal Thermal Energy Storage. Appl. Sci. 2019, 9, 267. https://doi.org/10.3390/app9020267
Duquesne M, Palomo Del Barrio E, Godin A. Nucleation Triggering of Highly Undercooled Xylitol Using an Air Lift Reactor for Seasonal Thermal Energy Storage. Applied Sciences. 2019; 9(2):267. https://doi.org/10.3390/app9020267
Chicago/Turabian StyleDuquesne, Marie, Elena Palomo Del Barrio, and Alexandre Godin. 2019. "Nucleation Triggering of Highly Undercooled Xylitol Using an Air Lift Reactor for Seasonal Thermal Energy Storage" Applied Sciences 9, no. 2: 267. https://doi.org/10.3390/app9020267
APA StyleDuquesne, M., Palomo Del Barrio, E., & Godin, A. (2019). Nucleation Triggering of Highly Undercooled Xylitol Using an Air Lift Reactor for Seasonal Thermal Energy Storage. Applied Sciences, 9(2), 267. https://doi.org/10.3390/app9020267