Synthesis of Poly(methyl methacrylate) Grafted Multiwalled Carbon Nanotubes via a Combination of RAFT and Alkyne-Azide Click Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Azide-Terminated Poly(methyl methacrylate) (PMMA)
2.3. Alkyne-Modification of MWCNT (MWCNTs-alkyne)
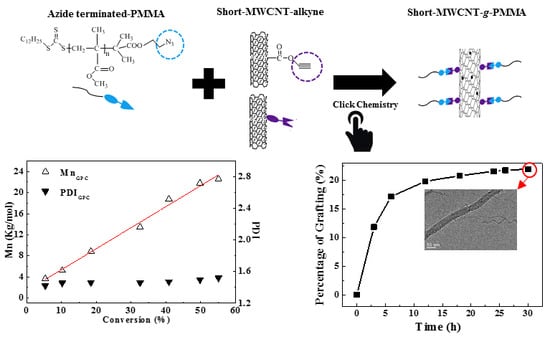
2.4. Preparation of MWCNTs-g-PMMA
2.5. Characterization
3. Results and Discussions
3.1. RAFT Polymerization of Azide-Terminated PMMA
3.2. TGA Analysis for the MWCNTS-g-PMMA
3.3. Surface Structure Analysis for MWCNTS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qu, X.L.; Alvarez, P.J.J.; Li, Q.L. Applications of nanotechnology in water and wastewater treatment. Water Resour. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.T. The impact of nanoscience on heterogeneous catalysis. Science 2003, 299, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Waghe, A.; Rasaiah, J.C.; Hummer, G. Filling and emptying kinetics of carbon nanotubes in water. J. Chem. Phys. 2002, 117, 10789–10795. [Google Scholar] [CrossRef]
- Holt, J.K.; Park, H.G.; Wang, Y.M.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Chopra, N.; Andrews, R.; Hinds, B.J. Nanoscale hydrodynamics—Enhanced flow in carbon nanotubes. Nature 2005, 438, 44. [Google Scholar] [CrossRef]
- Tunuguntla, R.H.; Henley, R.Y.; Yao, Y.C.; Pham, T.A.; Wanunu, M.; Noy, A. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 2017, 357, 792–796. [Google Scholar] [CrossRef]
- Liu, Z.P.; Yang, R. Synergistically-enhanced thermal conductivity of shape-stabilized phase change materials by expanded graphite and carbon nanotube. Appl. Sci. 2017, 7, 574. [Google Scholar] [CrossRef]
- Ma, W.; Gong, F.; Liu, C.; Tao, G.; Xu, J.; Jiang, B. SiO2 reinforced HDPE hybrid materials obtained by the sol–gel method. J. Appl. Polym. Sci. 2014, 131, 596–602. [Google Scholar] [CrossRef]
- Giovino, M.; Pribyl, J.; Benicewicz, B.; Kumar, S.; Schadler, L. Linear rheology of polymer nanocomposites with polymer-grafted nanoparticles. Polymer 2017, 131, 104–110. [Google Scholar] [CrossRef]
- Fontananova, E.; Bahattab, M.A.; Aljlil, S.A.; Alowairdy, M.; Rinaldi, G.; Vuono, D.; Nagy, J.B.; Drioli, E.; Di Profio, G. From hydrophobic to hydrophilic polyvinylidenefluoride (PVDF) membranes by gaining new insight into material’s properties. RSC Adv. 2015, 5, 56219–56231. [Google Scholar] [CrossRef]
- Li, H.B.; Shi, W.Y.; Su, Y.H.; Zhang, H.X.; Qin, X.H. Preparation and characterization of carboxylated multiwalled carbon nanotube/polyamide composite nanofiltration membranes with improved performance. J. Appl. Polym. Sci. 2017, 134, e45268. [Google Scholar] [CrossRef]
- Sanip, S.M.; Ismail, A.F.; Goh, P.S.; Soga, T.; Tanemura, M.; Yasuhiko, H. Gas separation properties of functionalized carbon nanotubes mixed matrix membranes. Sep. Purif. Technol. 2011, 78, 208–213. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Zdyrko, B.; Luzinov, I. Polymer brushes by the “grafting to” method. Macromol. Rapid. Comm. 2011, 32, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhou, B.; Liu, T.; Zhang, J.; Wang, X. The supramolecular organization of PVDF lamellae formed in diphenyl ketone dilutions via thermally induced phase separation. Colloid Polym. Sci. 2013, 291, 981–992. [Google Scholar] [CrossRef]
- Wu, P.; Feldman, A.K.; Nugent, A.K.; Hawker, C.J.; Scheel, A.; Voit, B.; Pyun, J.; Frechet, J.M.J.; Sharpless, K.B.; Fokin, V.V. Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper(I)-catalyzed ligation of azides and alkynes. Angew. Chem. Int. Ed. 2004, 43, 3928–3932. [Google Scholar] [CrossRef] [PubMed]
- Binder, W.H.; Sachsenhofer, R. ‘Click’ chemistry in polymer and materials science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Escorihuela, J.; Marcelis, A.T.; Zuilhof, H. Metal-free click chemistry reactions on surfaces. Adv. Mater. Interfaces 2015, 2, 1500135. [Google Scholar]
- Barner-Kowollik, C.; Du Prez, F.E.; Espeel, P.; Hawker, C.J.; Junkers, T.; Schlaad, H.; Camp, W.V. “Clicking” polymers or just efficient linking: What is the difference? Ang. Chem. Int. Ed. 2011, 50, 60–62. [Google Scholar] [CrossRef]
- Zhao, L.J.; Zhao, F.Q.; Zeng, B.Z. Synthesis of water-compatible surface-imprinted polymer via click chemistry and RAFT precipitation polymerization for highly selective and sensitive electrochemical assay of fenitrothion. Biosens. Bioelectron. 2014, 62, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, N.B.; Singha, N.K. Direct functionalization of multi-walled carbon nanotubes (MWCNTs) via grafting of poly(furfuryl methacrylate) using Diels-Alder “click chemistry” and its thermoreversibility. RSC Adv. 2015, 5, 94321–94327. [Google Scholar] [CrossRef]
- Gondi, S.R.; Vogt, A.P.; Sumerlin, B.S. Versatile pathway to functional telechelics via RAFT polymerization and click chemistry. Macromolecules 2007, 40, 474–481. [Google Scholar] [CrossRef]
- Ma, W.Z.; Zhao, Y.C.; Li, Y.X.; Zhang, P.; Cao, Z.; Yang, H.C.; Liu, C.L.; Tao, G.L.; Gong, F.H.; Matsuyama, H. Synthesis of hydrophilic carbon nanotubes by grafting poly(methyl methacrylate) via click reaction and its effect on poly(vinylidene fluoride)-carbon nanotube composite membrane properties. Appl. Surf. Sci. 2018, 435, 79–90. [Google Scholar] [CrossRef]
- Can, A.; Altuntas, E.; Hoogenboom, R.; Schubert, U.S. Synthesis and MALDI-TOF-MS of PS-PMA and PMA-PS block copolymers. Eur. Polym. J. 2010, 46, 1932–1939. [Google Scholar] [CrossRef]
- Chen, J.C.; Liu, M.Z.; Chen, C.; Gong, H.H.; Gao, C.M. Synthesis and characterization of silica nanoparticles with well-defined thermoresponsive PNIPAM via a combination of RAFT and click chemistry. ACS Appl. Mater. Interfaces 2011, 3, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.J.; Xu, Y.; Zhao, X.; Zhang, Q.H.; Chen, D.J. Grafting poly(methyl methacrylate) onto polyimide nanofibers via “click” reaction. ACS Appl. Mater. Interfaces 2009, 1, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Osswald, S.; Havel, M.; Gogotsi, Y. Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy. J. Raman Spectrosc. 2007, 38, 728–736. [Google Scholar] [CrossRef] [Green Version]
- Jorio, A.; Dresselhaus, G.; Dresselhaus, M.S.; Souza, M.; Dantas, M.S.S.; Pimenta, M.A.; Rao, A.M.; Saito, R.; Liu, C.; Cheng, H.M. Polarized Raman study of single-wall semiconducting carbon nanotubes. Phys. Rev. Lett. 2000, 85, 2617–2620. [Google Scholar] [CrossRef]
- Svrcek, V.; Pham-Huu, C.; Amadou, J.; Begin, D.; Ledoux, M.-J.; Le Normand, F.; Ersen, O.; Joulie, S. Filling and capping multiwall carbon nanotubes with silicon nanocrystals dispersed in SiO2-based spin on glass. J. Appl. Phys. 2006, 99, 064306. [Google Scholar] [CrossRef]
- Hu, C.C.; Su, J.H.; Wen, T.C. Modification of multi-walled carbon nanotubes for electric double-layer capacitors: Tube opening and surface functionalization. J. Phys. Chem. Solids 2007, 68, 2353–2362. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Zhao, Y.; Zhu, Z.; Guo, L.; Cao, Z.; Xia, Y.; Yang, H.; Gong, F.; Zhong, J. Synthesis of Poly(methyl methacrylate) Grafted Multiwalled Carbon Nanotubes via a Combination of RAFT and Alkyne-Azide Click Reaction. Appl. Sci. 2019, 9, 603. https://doi.org/10.3390/app9030603
Ma W, Zhao Y, Zhu Z, Guo L, Cao Z, Xia Y, Yang H, Gong F, Zhong J. Synthesis of Poly(methyl methacrylate) Grafted Multiwalled Carbon Nanotubes via a Combination of RAFT and Alkyne-Azide Click Reaction. Applied Sciences. 2019; 9(3):603. https://doi.org/10.3390/app9030603
Chicago/Turabian StyleMa, Wenzhong, Yuchen Zhao, Zhiwei Zhu, Lingxiang Guo, Zheng Cao, Yanping Xia, Haicun Yang, Fanghong Gong, and Jing Zhong. 2019. "Synthesis of Poly(methyl methacrylate) Grafted Multiwalled Carbon Nanotubes via a Combination of RAFT and Alkyne-Azide Click Reaction" Applied Sciences 9, no. 3: 603. https://doi.org/10.3390/app9030603
APA StyleMa, W., Zhao, Y., Zhu, Z., Guo, L., Cao, Z., Xia, Y., Yang, H., Gong, F., & Zhong, J. (2019). Synthesis of Poly(methyl methacrylate) Grafted Multiwalled Carbon Nanotubes via a Combination of RAFT and Alkyne-Azide Click Reaction. Applied Sciences, 9(3), 603. https://doi.org/10.3390/app9030603