Waste Bricks Applied as Removal Agent of Basic Blue 41 from Aqueous Solutions: Base Treatment and Their Regeneration Efficiency
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Basic Blue-41 Removal Experiments
2.3. Regeneration Tests
2.4. Characterization
3. Results and Discussion
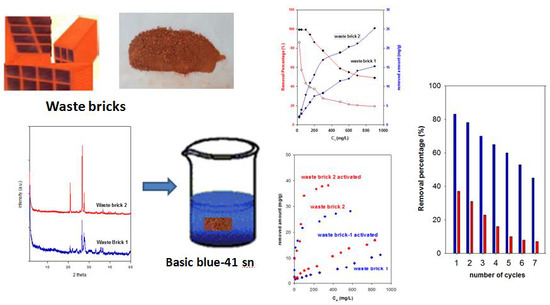
3.1. Characterization of the Used WB.
3.2. Removal of Basic Blue Properties
3.2.1. Effect of Initial Concentration
3.2.2. Effect of WB Mass
3.2.3. Effect of Removal Temperatures
3.2.4. Effect of the pH of the Basic Blue-41 Solution
3.2.5. Effect of WB Modification
3.3. Langmuir Isotherm and Maximum Removal Capacity
3.4. Regeneration Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gleick, P.H. An introduction to global fresh water issues. In Water in Crisis; Gleick, P.H., Ed.; Oxford University Press: New York, NY, USA, 1993; pp. 3–12. [Google Scholar]
- Somlyody, L.; Varis, O. Freshwater under pressure. Int. Rev. Environ. Strateg. 2006, 6, 181–204. [Google Scholar]
- Bixio, D.; Thoeye, C.; Wintgens, T.; Ravazzini, A.; Miska, V.; Muston, M.; Chikurel, H.; Aharoni, A.; Joksimovic, D.; Melin, T. Water reclamation and reuse: Implementation and management issues. Desalination 2008, 218, 13–23. [Google Scholar] [CrossRef]
- Baughman, G.L.; Perenich, T.A. Fate of dyes in aquatic systems: I. Solubility and partitioning of some hydrophobic dyes and related compounds. Environ. J. Toxicol. Chem. 1988, 7, 183–199. [Google Scholar] [CrossRef]
- Bae, J.S.; Freeman, H.S. Aquatic toxicity evaluation of new direct dyes to the Daphnia magna. Dyes Pigment. 2007, 73, 81–85. [Google Scholar] [CrossRef]
- De Sousa, M.L.; de Moraes, P.B.; Matos Lopes, P.R.; Montagnolli, R.N.; de Angelis, D.d.F.; Bidoia, E.D. Contamination by remazol red brilliant dye and its impact in aquatic photosynthetic microbiota. Environ. Mag. Sustain. Dev. 2012, 1, 129–138. [Google Scholar]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Garg, V.K.; Gupta, R.; Yadav, B.; Kumar, R. Removal of acid dyes by low cost adsorbent. Bioresour. Technol. 2003, 89, 121–124. [Google Scholar] [CrossRef]
- Vital, R.K.; Saibaba, K.V.N.; Shaik, K.B. Dye Removal by Adsorption: A Review. J. Bioremediat. Biodegrad. 2016, 7, 371–374. [Google Scholar]
- Sanghi, R.; Bhattacharaya, B. Review on decolourization of aqueous dye solution by low cost adsorbents. Color. Technol. 2002, 118, 256–269. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Review on Dye Removal from Its Aqueous Solution into Alternative Cost Effective and Non-Conventional Adsorbents. J. Chem. Process. Eng. 2014, 1, 1–7. [Google Scholar]
- Benalia, A.; Derbal, K.; Panico, A.; Piroz, F. Use of Acorn Leaves as a natural coagulant in a drinking water treatment plant. Water 2019, 11, 57. [Google Scholar] [CrossRef]
- Hu, Q.H.; Qiao, S.Z.; Haghseresht, F. Adsorption study for the removal of basic red dye using bentonite. Ind. Eng. Chem. Res. 2006, 45, 733–738. [Google Scholar] [CrossRef]
- Dogan, M.; Karaoglu, M.H.; Alkan, M. Adsorption kinetics of maxillon yellow 4GL and maxilon red GRL dyes on kaolinite. J. Hazard. Mater. 2009, 165, 1142–1151. [Google Scholar] [CrossRef]
- Adeyemo, D.A.; Adeoye, I.O.; Bello, O.S. Adsorption of dyes using different types of clay: A review. Appl. Water Sci. 2017, 7, 543–568. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Abu-Aitah, B.F. The removal of methylene blue dye using activated and non-activated bentonite solutions. Adsorpt. Sci. Technol. 2003, 21, 451–462. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chiang, C.C.; Yu, M.F. Adsorption behavior of basic dyes on activated clay. Sep. Sci. Technol. 1997, 32, 2513–2534. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Al-Faze, R.; Al Suhaimi, A. Effect of acid activation of Saudi local clay mineral on removal properties of basic blue 41 from an aqueous solution. Appl. Clay Sci. 2015, 116–117, 23–30. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Abboudi, M.; Rakass, S.; Oudghiri Hassani, H.; Ibrahim, S.M.; Al-Faze, R. Removal Properties of Anionic Dye Eosin by Cetyltrimethylammonium Organo-Clays: The Effect of Counter-Ions and Regeneration Studies. Molecules 2018, 23, 2364. [Google Scholar] [CrossRef]
- Gil, A.; Assis, F.C.C.; Albeniz, S.; Korili, S.A. Removal of dyes from wastewaters by adsorption on pillared clays. Chem. Eng. J. 2011, 168, 1032–1040. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Removal of Crystal Violet dye from aqueous solution using calcined and uncalcined mixed clay adsorbents. Sep. Sci. Technol. 2009, 45, 94–104. [Google Scholar] [CrossRef]
- Vimonses, V.; Jin, B.; Chow, C.W.K.; Saint, C. Enhancing removal efficiency of anionic dye by combination and calcination of clay materials and calcium hydroxide. J. Hazard. Mater. 2009, 171, 941–947. [Google Scholar] [CrossRef]
- Momina; Shahadat, M.; Isamil, S. Regeneration performance of clay-based adsorbents for the removal of industrial dyes: A review. RSC Adv. 2018, 8, 24571–24587. [Google Scholar] [CrossRef]
- Cheng, H. Reuse research progress on waste clay brick. Procedia Environ. Sci. 2016, 31, 218–226. [Google Scholar] [CrossRef]
- Demir, I.; Orhan, M. Reuse of waste bricks in the production line. Build. Environ. 2003, 38, 1451–1455. [Google Scholar] [CrossRef]
- Naceri, A.; Hamina, M.C. Use of waste brick as a partial replacement of cement in mortar. J. Waste Manag. 2009, 29, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- Labidi, N.S. Removal of Mercury from Aqueous Solutions by Waste Brick. Int. J. Environ. Res. 2008, 2, 275–278. [Google Scholar]
- Yadav, A.K.; Kaushik, C.P.; Haritash, A.K.; Kansal, A.; Rani, N. Defluoridation of groundwater using brick powder as an adsorbent. J. Hazard. Mater. 2006, 128, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Dai, Y.; Chang, J.; Wu, C.; Wu, Z.; Liang, W. Adsorption characteristics of used brick for phosphorus removal from phosphate solution. Desalin. Water Treat. 2013, 51, 5886–5891. [Google Scholar] [CrossRef] [Green Version]
- Hamdaoui, O. Batch Study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J. Hazard. Mater. 2006, B135, 264–273. [Google Scholar] [CrossRef]
- El-Shahat, M.F.; Shehata, A.M.A. Adsorption of lead, cadmium and zinc ions from industrial wastewater by using raw clay and broken clay-brick waste. Asian J. Chem. 2013, 25, 4284–4288. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Chen, H.; Zhang, X.; Li, H. The systematic adsorption of diclofenac onto waste red bricks functionalized with iron oxides. Water 2018, 10, 1343. [Google Scholar] [CrossRef]
- Kooli, F.; Yan, L.; Al-Faze, R.; Al-Sehimi, A. Removal enhancement of basic blue 41 by waste brick from an aqueous solution. Arab. J. Chem. 2015, 8, 333–342. [Google Scholar] [CrossRef]
- Dehou, C.; Wartel, M.; Recourt, P.; Revel, B.; Mabingui, J.; Montiel, A.; Boughriet, A. Physicochemical crystalline and morphological characteristics of bricks used for ground waters purification in Bangui region (Central African Republic). Appl. Clay Sci. 2012, 59, 69–75. [Google Scholar] [CrossRef]
- Cheng, H.P.; Huanga, Y.H.; Leeb, C. Decolorization of reactive dye using a photo-ferrioxalate system with brick grain-supported iron oxide. J. Hazard. Mater. 2011, 188, 357–362. [Google Scholar] [CrossRef]
- Fatimah, I.; Fadillah, S. TiO2 supported on brick waste as low cost photocatalyst for dye photodegradation. Chem. Eng. Trans. 2018, 63, 733–738. [Google Scholar]
- Rouquerol, J.; Llewellyn, P.; Sing, K. Adsorption by Clays, Pillared Clays, Zeolites and Aluminophosphates; Springer: Oxford, UK, 2014; pp. 467–527. [Google Scholar]
- Aboudi Mana, S.C.; Hanafiah, M.M.; Chowdhury, A.J.K. Environmental characteristics of clay and clay-based minerals. Geol. Ecol. Landsc. 2017, 1, 155–161. [Google Scholar] [CrossRef]
- Pires, J.; Machado, M.; de Carvalho, M.B. Porosity and thermal stability of PILCs prepared with clays from different origins and different metal–polyhydroxycationic species ofAl and Al/Ce. J. Mater. Chem. 1998, 8, 1465–1469. [Google Scholar] [CrossRef]
- Munoz, H.J.; Blanco, C.; Gil, A.; VVicente, M.A.; Galeano, L.A. Preparation of Al/Fe-Pillared Clays: Effect of the Starting Mineral. Materials 2017, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Tsozué, D.; Nzeugang, A.N.; Mache, J.R.; Loweh, S.; Fagel, N. Mineralogical, physico-chemical and technological characterization of clays from Maroua (Far-North, Cameroon) for use in ceramic bricks production. J. Build. Eng. 2017, 11, 17–24. [Google Scholar] [CrossRef]
- Chin, C.L.; Ahmad, Z.A.; Sow, S.S. Relationship between the thermal behaviour of the clays and their mineralogical and chemical composition: Example of Ipoh, Kuala Rompin and Mersing (Malaysia). Appl. Clay Sci. 2017, 143, 327–335. [Google Scholar] [CrossRef]
- Campbell, J.W.P.; Pryce, W. Brick: A World History; Thames and Hudson: New York, NY, USA, 2003. [Google Scholar]
- Hussin, F.; Aroua, M.K.; Daud, W.M.A.W. Textural characteristics, surface chemistry and activation of bleaching earth: A review. Chem. Eng. J. 2011, 170, 90–106. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Tan, S.X.; Zheng, J. Organoclays from alkaline-treated acid-activated clays. J. Therm. Anal. Calorim. 2014, 115, 1465–1475. [Google Scholar] [CrossRef]
- Song, J.G.; Wang, F.; Bai, X.B.; Du, D.M.; Ju, Y.Y.; Xu, M.H.; Ji, G.-C. Effect of the sintering technology on the properties of fired brick from quartz sand. J. Ceram. Proc. Res. 2011, 12, 357–360. [Google Scholar]
- Russell, J.D. A Hand Book of Determinative Methods in Clay Mineralogy; Wilson, M.J., Ed.; Blackie and Son Ltd.: Glasgow, UK, 1987. [Google Scholar]
- Percival, H.J.; Duncan, J.F.; Fosters, P.K. Interpretation of the Kaolinite-Mullite Reaction Sequence from Infrared Absorption Spectra. J. Am. Ceram. Soc. 1974, 57, 57–64. [Google Scholar] [CrossRef]
- Nirmala, G.; Viruthagiri, G. FT-IR characterization of articulated ceramic bricks with wastes from ceramic industries. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 126, 129–134. [Google Scholar] [CrossRef]
- Addadi, L.; Raz, S.; Weiner, S. Taking advantage of disorder: Amorphous calcium carbonate and its role in biomineralization. Adv. Mater. 2003, 15, 960–970. [Google Scholar] [CrossRef]
- Ghosh, S.N. Infra-red spectra of some selected minerals, rocks and products. J. Mater. Sci. 1978, 13, 1877–1886. [Google Scholar] [CrossRef]
- Khouzani, M.F.; Chevrier, D.M.; Güttlein, P.; Hauser, K.; Zhang, P.; Hedin, N.; Gebauer, D. Disordered amorphous calcium carbonate from direct precipitation. Cryst. Eng. Comm. 2015, 17, 4842–4849. [Google Scholar] [CrossRef] [Green Version]
- Dehou, S.C.; Wartel, M.; Recourt, P.; Revel, B.; Boughriet, A. Acid Treatment of Crushed Brick (from Central African Republic) and Its Ability (after FeOOH Coating) to Adsorb Ferrous Ions from Aqueous Solutions. Open Mater. Sci. J. 2012, 6, 50–59. [Google Scholar] [CrossRef]
- Reyad, A.; Shawabkeh, R.A.; Tutunji, M.F. Experimental study and modeling of basic dye sorption by diatomaceous clay. Appl. Clay Sci. 2003, 24, 111–120. [Google Scholar]
- Khraisheh, M.A.; Al-Ghouti, M.A.; Allen, S.J.; Ahmad, M.N. The effect of pH, temperature, and molecular size on the removal of dyes from textile effluent using manganese oxides-modified diatomite. Water Environ. Res. 2004, 76, 2655–2663. [Google Scholar] [CrossRef]
- Shahryari, Z.; Goharrizi, A.S.; Azadi, M. Experimental study of methylene blue adsorption from aqueous solutions onto carbon nanotubes. Int. J. Water Resour. Environ. Eng. 2010, 2, 216–280. [Google Scholar]
- Sharma, P.; Kaur, R.; Baskar, C.; Chung, W.J. Removal of methylene blue from aqueous waste using rice husk and rice husk ash. Desalination 2010, 259, 249–257. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Hbaieb, K.; Ching, O.Y.; Al-Faze, R. Characterization of organo-kenyaites: thermal stability and their effects on eosin removal characteristics. Clay Miner. 2018, 53, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Regti, A.; Laamari, M.R.; Stiriba, S.; El Haddad, M. Removal of Basic Blue 41 dyes using Persea americana-activated carbon prepared by phosphoric acid action. Int. J. Ind. Chem. 2017, 8, 187–195. [Google Scholar] [CrossRef]
- Mehrizi, M.Z.; Badiei, A. Highly efficient removal of Basic Blue 41 with nanoporous silica. Water Resour. Ind. 2014, 5, 49–57. [Google Scholar] [CrossRef]
- Abul Hossain, M.; Mohibullah, M. Kinetics and thermodynamics of adsoprtion of basic Blue 41 on used black tea leaves. Int. J. Sci. Res. 2017, 8, 995–1002. [Google Scholar]
- Meroufel, B.; Benali, O.; Benyahia, M.; Benmoussa, Y.; Zenasni, M.A. Adsorptive removal of anionic dye from aqueous solutions by Algerian kaolin: Characteristics, isotherm, and thermodynamic studies. J. Mater. Environ. Sci. 2013, 4, 482–491. [Google Scholar]
- Gupta, V.K.; Jain, R.; Siddiqui, M.N.; Saleh, T.A.; Agarwal, S.; Malati, S.; Pathak, D. Equilibrium and thermodynamic studies on the adsorption of the dye. J. Chem. Eng. Data 2010, 55, 5225–5229. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.J. Biosorption isotherms, kinetics and thermodynamics. Sep. Purif. Technol. 2008, 61, 229–242. [Google Scholar] [CrossRef]
- Ma, J.; Jia, Y.; Jing, Y.; Yao, Y.; Sun, J. Kinetics and thermodynamics of methylene blue adsorption by cobalt-hectorite composite. Dyes Pigment. 2012, 93, 1441–1446. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.U. Anionic dye removal from aqueous solutions using modified zeolite: Adsorption kinetics and isotherm studies. Chem. Eng. J. 2012, 200–202, 59–67. [Google Scholar] [CrossRef]
- Humelnicu, I.; Baiceanu, A.; Ignat, M.E.; Dulman, V. The removal of basic blue 41 textile dye from aqueous solution by adsorption onto natural zeolitic tuff: Kinetics and thermodynamics. Process Saf. Environ. Prot. 2017, 105, 274–287. [Google Scholar] [CrossRef]
- Atar, M.; Olgun, A.; Colak, F. Thermodynamic, equilibrium and kinetic study of the biosorption of Basic Blue 41 using Bacillus macerans. Eng. Life Sci. 2008, 8, 499–506. [Google Scholar] [CrossRef]
- Roulia, M.; Vassiliadis, A.A. Interactions between C.I. basic blue 41 and aluminosilicate sorbents. J. Colloid Interface Sci. 2005, 292, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.N.; Ram, R.N. Removal of basic dye from waste-water using silica as adsorbent. Environ. Pollut. 1992, 77, 79–86. [Google Scholar] [CrossRef]
- Dove, P.M.; Rimstidt, J.D. Silica-water interactions. Silica reviews in Mineralogy. Miner. Soc. Am. 1994, 29, 259–307. [Google Scholar]
- Gougazeh, M.; Kooli, F.; Buhl, J.C. Removal efficiency of basicblue41 by three zeolites prepared from natural Jordanian kaolinite. Accept. Clays Clay Miner. 2019. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 39, 2221–2295. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, J.; Ge, F.; Yuan, P. Regeneration of spent organoclays after the soprtion of organic pollutants: A review. J. Environ. Manag. 2009, 90, 3212–3216. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F.; Liu, Y.; Abboudi, M.; Rakass, S.; Oudghiri Hassani, H.; Ibrahim, S.M.; Al-Faze, R. Application of Organo-Magadiites for the Removal of Eosin Dye from Aqueous Solutions: Thermal Treatment and Regeneration. Molecules 2018, 23, 2280. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D.; Gonzalez, M.A. Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions. Environ. Sci. Technol. 2006, 40, 1000–1007. [Google Scholar] [CrossRef] [PubMed]









| Samples | SiO2 | Al2O3 | MgO | Fe2O3 | CaO | K2O | Na2O |
|---|---|---|---|---|---|---|---|
| Jed-WB | 58.28 | 23.72 | 1.53 | 12.32 | 2.43 | 0.89 | 0.72 |
| Med-WB | 54.60 | 17.57 | 4.48 | 9.42 | 7.40 | 2.53 | 2.19 |
| B-Med * | 54.88 | 17.24 | 4.70 | 9.70 | 7.31 | 2.43 | 2.10 |
| B-Jed * | 57.54 | 23.41 | 1.32 | 12.34 | 2.38 | 0.56 | 0.53 |
| Samples | SBET (m2/g) | P.V (cc/g) | A.P.D. (nm) |
|---|---|---|---|
| Jed-WB | 1.00 | 0.001 | 7.81 |
| Med-WB | 2.76 | 0.005 | 7.95 |
| B-Med * | 3.77 | 0.003 | 4.60 |
| B-Jed * | 4.42 | 0.014 | 12.80 |
| Materials | ∆H° (kJ·mol−1) | ∆S° (kJ·mol−1·K) | ∆G° (kJ·mol−1) | ||
|---|---|---|---|---|---|
| Med-WB | 135.12 | 0.45 | 303 K | 323 K | 333 K |
| −1.49 | −3.59 | −17.05 | |||
| Jed-WB | 43.92 | 0.17 | −0.74 | −1.36 | −4.80 |
| Samples | qm (mg·g−1) | KL (L·g−1) | R2 |
|---|---|---|---|
| Med-WB | 29.01 | 0.096 | 0.9932 |
| Jed-WB | 16.81 | 0.007 | 0.9861 |
| Med-WB-30 °C | 32.15 | 0.234 | 0.9885 |
| Jed-WB-30 °C | 20.83 | 0.004 | 0.9787 |
| Med-WB-50 °C | 34.89 | 0.256 | 0.9861 |
| Jed-WB-50 °C | 30.03 | 0.060 | 0.9932 |
| Med-WB-60 °C | 58.47 | 0.439 | 0.9975 |
| Jed-WB-60 °C | 36.40 | 0.053 | 0.9767 |
| B-Med-WB | 43.50 | 0.210 | 0.9962 |
| B-Jed-WB | 34.36 | 0.029 | 0.9654 |
| Samples | qmax (mg/g) | Reference |
|---|---|---|
| Med-WB | 30.03 | [33] |
| Jed-WB | 16.80 | This study |
| B-Med-WB | 43.50 | [33] |
| B-Jed-WB | 34.36 | This study |
| Kaolinite | 6.20 | [73] |
| Hydroxysodalite | 39.37 | [73] |
| Zeolite –X | 17.69 | [73] |
| Zeolite Y | 26.80 | [73] |
| Local Clay | 73.00 | [19] |
| Acid-activated local clays | 50.34 | [19] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kooli, F.; Liu, Y.; Abboudi, M.; Oudghiri Hassani, H.; Rakass, S.; Ibrahim, S.M.; Al Wadaani, F. Waste Bricks Applied as Removal Agent of Basic Blue 41 from Aqueous Solutions: Base Treatment and Their Regeneration Efficiency. Appl. Sci. 2019, 9, 1237. https://doi.org/10.3390/app9061237
Kooli F, Liu Y, Abboudi M, Oudghiri Hassani H, Rakass S, Ibrahim SM, Al Wadaani F. Waste Bricks Applied as Removal Agent of Basic Blue 41 from Aqueous Solutions: Base Treatment and Their Regeneration Efficiency. Applied Sciences. 2019; 9(6):1237. https://doi.org/10.3390/app9061237
Chicago/Turabian StyleKooli, Fethi, Yan Liu, Mostafa Abboudi, Hicham Oudghiri Hassani, Souad Rakass, Sheikh Muhammad Ibrahim, and Fahd Al Wadaani. 2019. "Waste Bricks Applied as Removal Agent of Basic Blue 41 from Aqueous Solutions: Base Treatment and Their Regeneration Efficiency" Applied Sciences 9, no. 6: 1237. https://doi.org/10.3390/app9061237
APA StyleKooli, F., Liu, Y., Abboudi, M., Oudghiri Hassani, H., Rakass, S., Ibrahim, S. M., & Al Wadaani, F. (2019). Waste Bricks Applied as Removal Agent of Basic Blue 41 from Aqueous Solutions: Base Treatment and Their Regeneration Efficiency. Applied Sciences, 9(6), 1237. https://doi.org/10.3390/app9061237