BDNF and Pro-BDNF in Amyotrophic Lateral Sclerosis: A New Perspective for Biomarkers of Neurodegeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Serum and CSF Sampling
2.3. ELISA Assays
2.4. Simoa Assay
2.5. Statistical Analysis
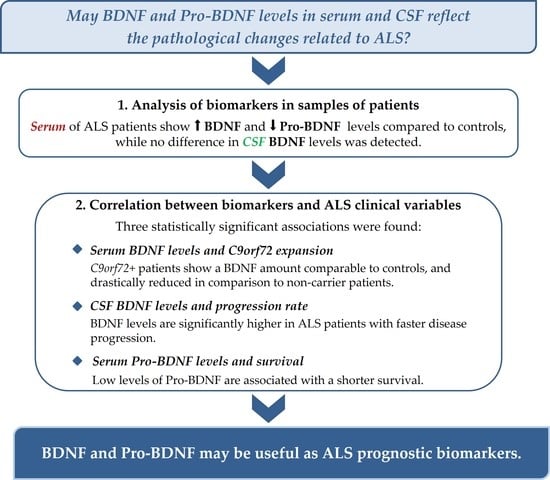
3. Results
3.1. BDNF and Pro-BDNF in Serum
3.2. BDNF in CSF
3.3. Correlation with ALS Clinical Variables
3.3.1. Serum
3.3.2. CSF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, P. Amyotrophic Lateral Sclerosis: An Update for 2013 Clinical Features, Pathophysiology, Management and Therapeutic Trials. Aging Dis. 2013, 4, 295–310. [Google Scholar] [CrossRef]
- Hulisz, D. Amyotrophic Lateral Sclerosis: Disease State Overview. Am. J. Manag. Care 2018, 24 (Suppl. S15), S320–S326. [Google Scholar]
- Gromicho, M.; Figueiral, M.; Uysal, H.; Grosskreutz, J.; Kuzma-Kozakiewicz, M.; Pinto, S.; Petri, S.; Madeira, S.; Swash, M.; Carvalho, M. Spreading in ALS: The Relative Impact of Upper and Lower Motor Neuron Involvement. Ann. Clin. Transl. Neurol. 2020, 7, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, J.; Noakes, P.G.; Bellingham, M.C. The Role of Altered BDNF/TrkB Signaling in Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2019, 13, 368. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, T.; Liu, L.; Yao, X.; Chen, L.; Fan, D.; Zhan, S.; Wang, S. Global Variation in Prevalence and Incidence of Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. J. Neurol. 2020, 267, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef] [Green Version]
- Volk, A.E.; Weishaupt, J.H.; Andersen, P.M.; Ludolph, A.C.; Kubisch, C. Current Knowledge and Recent Insights into the Genetic Basis of Amyotrophic Lateral Sclerosis. Med. Genet. 2018, 30, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Sabatelli, M.; Conte, A.; Zollino, M. Clinical and Genetic Heterogeneity of Amyotrophic Lateral Sclerosis: Clinical and Genetic Heterogeneity of ALS. Clin. Genet. 2013, 83, 408–416. [Google Scholar] [CrossRef]
- Bonafede, R.; Mariotti, R. ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles. Front. Cell. Neurosci. 2017, 11, 80. [Google Scholar] [CrossRef]
- Luo, L.; Li, C.; Du, X.; Shi, Q.; Huang, Q.; Xu, X.; Wang, Q. Effect of Aerobic Exercise on BDNF/ProBDNF Expression in the Ischemic Hippocampus and Depression Recovery of Rats after Stroke. Behav. Brain Res. 2019, 362, 323–331. [Google Scholar] [CrossRef]
- Mattson, M.P.; Maudsley, S.; Martin, B. BDNF and 5-HT: A Dynamic Duo in Age-Related Neuronal Plasticity and Neurodegenerative Disorders. Trends Neurosci. 2004, 27, 589–594. [Google Scholar] [CrossRef]
- Murray, P.S.; Holmes, P.V. An Overview of Brain-Derived Neurotrophic Factor and Implications for Excitotoxic Vulnerability in the Hippocampus. Int. J. Pept. 2011, 2011, 654085. [Google Scholar] [CrossRef] [Green Version]
- Je, H.S.; Yang, F.; Ji, Y.; Nagappan, G.; Hempstead, B.L.; Lu, B. Role of Pro-Brain-Derived Neurotrophic Factor (ProBDNF) to Mature BDNF Conversion in Activity-Dependent Competition at Developing Neuromuscular Synapses. Proc. Natl. Acad. Sci. USA 2012, 109, 15924–15929. [Google Scholar] [CrossRef] [Green Version]
- Sasi, M.; Vignoli, B.; Canossa, M.; Blum, R. Neurobiology of Local and Intercellular BDNF Signaling. Pflüg. Arch.-Eur. J. Physiol. 2017, 469, 593–610. [Google Scholar] [CrossRef] [Green Version]
- Borodinova, A.A.; Salozhin, S.V. Differences in the Biological Functions of BDNF and ProBDNF in the Central Nervous System. Neurosci. Behav. Physiol. 2017, 47, 251–265. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Mini Review. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Levi-Montalcini, R. The Nerve Growth Factor 35 Years Later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Foltran, R.B.; Diaz, S.L. BDNF Isoforms: A Round Trip Ticket between Neurogenesis and Serotonin? J. Neurochem. 2016, 138, 204–221. [Google Scholar] [CrossRef]
- Lessmann, V.; Gottmann, K.; Malcangio, M. Neurotrophin Secretion: Current Facts and Future Prospects. Prog. Neurobiol. 2003, 69, 341–374. [Google Scholar] [CrossRef]
- De Vincenti, A.P.; Ríos, A.S.; Paratcha, G.; Ledda, F. Mechanisms That Modulate and Diversify BDNF Functions: Implications for Hippocampal Synaptic Plasticity. Front. Cell. Neurosci. 2019, 13, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Lim, Y.; Li, F.; Liu, S.; Lu, J.-J.; Haberberger, R.; Zhong, J.-H.; Zhou, X.-F. ProBDNF Collapses Neurite Outgrowth of Primary Neurons by Activating RhoA. PLoS ONE 2012, 7, e35883. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, L.F. Neurotrophin-Regulated Signalling Pathways. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, H.K. ProBDNF Induces Neuronal Apoptosis via Activation of a Receptor Complex of P75NTR and Sortilin. J. Neurosci. 2005, 25, 5455–5463. [Google Scholar] [CrossRef]
- Anastasia, A.; Deinhardt, K.; Chao, M.V.; Will, N.E.; Irmady, K.; Lee, F.S.; Hempstead, B.L.; Bracken, C. Val66Met Polymorphism of BDNF Alters Prodomain Structure to Induce Neuronal Growth Cone Retraction. Nat. Commun. 2013, 4, 2490. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, D.R.; Miller, F.D. Neurotrophin Signal Transduction in the Nervous System. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Gonzalez, A.; Moya-Alvarado, G.; Gonzalez-Billaut, C.; Bronfman, F.C. Cellular and Molecular Mechanisms Regulating Neuronal Growth by Brain-Derived Neurotrophic Factor: Neuronal Growth by Brain-Derived Neurotrophic Factor. Cytoskeleton 2016, 73, 612–628. [Google Scholar] [CrossRef] [Green Version]
- Bharani, K.L.; Ledreux, A.; Gilmore, A.; Carroll, S.L.; Granholm, A.-C. Serum Pro-BDNF Levels Correlate with Phospho-Tau Staining in Alzheimer’s Disease. Neurobiol. Aging 2020, 87, 49–59. [Google Scholar] [CrossRef]
- Yang, M.; Lim, Y.; Li, X.; Zhong, J.-H.; Zhou, X.-F. Precursor of Brain-Derived Neurotrophic Factor (ProBDNF) Forms a Complex with Huntingtin-Associated Protein-1 (HAP1) and Sortilin That Modulates ProBDNF Trafficking, Degradation, and Processing. J. Biol. Chem. 2011, 286, 16272–16284. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, R.; Matsumoto, M.; Fujikawa, A.; Kato, A.; Kuboyama, K.; Yonehara, K.; Shintani, T.; Sakuta, H.; Noda, M. SPIG1 Negatively Regulates BDNF Maturation. J. Neurosci. 2014, 34, 3429–3442. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L.; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial Revisited: Revised Criteria for the Diagnosis of Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. Mot. Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Kimura, F.; Fujimura, C.; Ishida, S.; Nakajima, H.; Furutama, D.; Uehara, H.; Shinoda, K.; Sugino, M.; Hanafusa, T. Progression Rate of ALSFRS-R at Time of Diagnosis Predicts Survival Time in ALS. Neurology 2006, 66, 265–267. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Tumani, H.; Engelborghs, S.; Mollenhauer, B. Biobanking of CSF: International Standardization to Optimize Biomarker Development. Clin. Biochem. 2014, 47, 288–292. [Google Scholar] [CrossRef]
- Lu, B.; Pang, P.T.; Woo, N.H. The Yin and Yang of Neurotrophin Action. Nat. Rev. Neurosci. 2005, 6, 603–614. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Xie, Y.; Qin, D. Proteolytic Cleavage of ProBDNF to MBDNF in Neuropsychiatric and Neurodegenerative Diseases. Brain Res. Bull. 2021, 166, 172–184. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of Brain-Derived Neurotrophic Factor across the Blood-Brain Barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Poduslo, J.F.; Curran, G.L. Permeability at the Blood-Brain and Blood-Nerve Barriers of the Neurotrophic Factors: NGF, CNTF, NT-3, BDNF. Brain Res. Mol. Brain Res. 1996, 36, 280–286. [Google Scholar] [CrossRef]
- Buck, C.R.; Seburn, K.L.; Cope, T.C. Neurotrophin Expression by Spinal Motoneurons in Adult and Developing Rats. J. Comp. Neurol. 2000, 416, 309–318. [Google Scholar] [CrossRef]
- Gravel, C.; Götz, R.; Lorrain, A.; Sendtner, M. Adenoviral Gene Transfer of Ciliary Neurotrophic Factor and Brain-Derived Neurotrophic Factor Leads to Long-Term Survival of Axotomized Motor Neurons. Nat. Med. 1997, 3, 765–770. [Google Scholar] [CrossRef]
- Josephson, A.; Widenfalk, J.; Trifunovski, A.; Widmer, H.R.; Olson, L.; Spenger, C. GDNF and NGF Family Members and Receptors in Human Fetal and Adult Spinal Cord and Dorsal Root Ganglia. J. Comp. Neurol. 2001, 440, 204–217. [Google Scholar] [CrossRef]
- Tovar-Y-Romo, L.B.; Ramírez-Jarquín, U.N.; Lazo-Gómez, R.; Tapia, R. Trophic Factors as Modulators of Motor Neuron Physiology and Survival: Implications for ALS Therapy. Front. Cell. Neurosci. 2014, 8, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iłzecka, J.; Stelmasiak, Z. Brain-Derived Neurotrophic Factor Is Not Altered in the Serum and Cerebrospinal Fluid of Amyotrophic Lateral Sclerosis Patients. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2002, 22, 473–474. [Google Scholar] [CrossRef]
- Tremolizzo, L.; Pellegrini, A.; Conti, E.; Arosio, A.; Gerardi, F.; Lunetta, C.; Magni, P.; Appollonio, I.; Ferrarese, C. BDNF Serum Levels with Respect to Multidimensional Assessment in Amyotrophic Lateral Sclerosis. Neurodegener. Dis. 2016, 16, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Grundström, E.; Lindholm, D.; Johansson, A.; Blennow, K.; Askmark, H. GDNF but Not BDNF Is Increased in Cerebrospinal Fluid in Amyotrophic Lateral Sclerosis. Neuroreport 2000, 11, 1781–1783. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Peskind, E.R.; Millard, S.P.; Chi, P.; Sokal, I.; Yu, C.-E.; Bekris, L.M.; Raskind, M.A.; Galasko, D.R.; Montine, T.J. Cerebrospinal Fluid Concentration of Brain-Derived Neurotrophic Factor and Cognitive Function in Non-Demented Subjects. PLoS ONE 2009, 4, e5424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forlenza, O.V.; Diniz, B.S.; Teixeira, A.L.; Ojopi, E.B.; Talib, L.L.; Mendonça, V.A.; Izzo, G.; Gattaz, W.F. Effect of Brain-Derived Neurotrophic Factor Val66Met Polymorphism and Serum Levels on the Progression of Mild Cognitive Impairment. World J. Biol. Psychiatry 2010, 11, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Pláteník, J.; Fišar, Z.; Buchal, R.; Jirák, R.; Kitzlerová, E.; Zvěřová, M.; Raboch, J. GSK3β, CREB, and BDNF in Peripheral Blood of Patients with Alzheimer’s Disease and Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 50, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Spalletta, G.; Di Iulio, F.; Ciaramella, A.; Salani, F.; Colantoni, L.; Varsi, A.E.; Gianni, W.; Sancesario, G.; Caltagirone, C.; et al. Alzheimer’s Disease (AD) and Mild Cognitive Impairment (MCI) Patients Are Characterized by Increased BDNF Serum Levels. Curr. Alzheimer Res. 2010, 7, 15–20. [Google Scholar] [CrossRef]
- Faria, M.C.; Gonçalves, G.S.; Rocha, N.P.; Moraes, E.N.; Bicalho, M.A.; Gualberto Cintra, M.T.; Jardim de Paula, J.; José Ravic de Miranda, L.F.; Clayton de Souza Ferreira, A.; Teixeira, A.L.; et al. Increased Plasma Levels of BDNF and Inflammatory Markers in Alzheimer’s Disease. J. Psychiatr. Res. 2014, 53, 166–172. [Google Scholar] [CrossRef]
- Nettiksimmons, J.; Simonsick, E.M.; Harris, T.; Satterfield, S.; Rosano, C.; Yaffe, K.; Health ABC Study. The Associations between Serum Brain-Derived Neurotrophic Factor, Potential Confounders, and Cognitive Decline: A Longitudinal Study. PLoS ONE 2014, 9, e91339. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-Derived Neurotrophic Factor in Alzheimer’s Disease and Its Pharmaceutical Potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Ng, T.; Ho, C.; Tam, W.; Kua, E.; Ho, R. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef] [Green Version]
- Le Blanc, J.; Fleury, S.; Boukhatem, I.; Bélanger, J.-C.; Welman, M.; Lordkipanidzé, M. Platelets Selectively Regulate the Release of BDNF, But Not That of Its Precursor Protein, ProBDNF. Front. Immunol. 2020, 11, 575607. [Google Scholar] [CrossRef]
- Burk, K.; Pasterkamp, R.J. Disrupted Neuronal Trafficking in Amyotrophic Lateral Sclerosis. Acta Neuropathol. 2019, 137, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Farg, M.A.; Sundaramoorthy, V.; Sultana, J.M.; Yang, S.; Atkinson, R.A.K.; Levina, V.; Halloran, M.A.; Gleeson, P.A.; Blair, I.P.; Soo, K.Y.; et al. C9ORF72, Implicated in Amytrophic Lateral Sclerosis and Frontotemporal Dementia, Regulates Endosomal Trafficking. Hum. Mol. Genet. 2014, 23, 3579–3595. [Google Scholar] [CrossRef]
- Bemelmans, A.-P.; Husson, I.; Jaquet, M.; Mallet, J.; Kosofsky, B.E.; Gressens, P. Lentiviral-Mediated Gene Transfer of Brain-Derived Neurotrophic Factor Is Neuroprotective in a Mouse Model of Neonatal Excitotoxic Challenge. J. Neurosci. Res. 2006, 83, 50–60. [Google Scholar] [CrossRef]
- Henriques, A.; Pitzer, C.; Schneider, A. Neurotrophic Growth Factors for the Treatment of Amyotrophic Lateral Sclerosis: Where Do We Stand? Front. Neurosci. 2010, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Shruthi, S.; Sumitha, R.; Varghese, A.M.; Ashok, S.; Chandrasekhar Sagar, B.K.; Sathyaprabha, T.N.; Nalini, A.; Kramer, B.W.; Raju, T.R.; Vijayalakshmi, K.; et al. Brain-Derived Neurotrophic Factor Facilitates Functional Recovery from ALS-Cerebral Spinal Fluid-Induced Neurodegenerative Changes in the NSC-34 Motor Neuron Cell Line. Neurodegener. Dis. 2017, 17, 44–58. [Google Scholar] [CrossRef]
- Fryer, H.J.; Wolf, D.H.; Knox, R.J.; Strittmatter, S.M.; Pennica, D.; O’Leary, R.M.; Russell, D.S.; Kalb, R.G. Brain-Derived Neurotrophic Factor Induces Excitotoxic Sensitivity in Cultured Embryonic Rat Spinal Motor Neurons through Activation of the Phosphatidylinositol 3-Kinase Pathway. J. Neurochem. 2000, 74, 582–595. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Kalb, R.G. BDNF Heightens the Sensitivity of Motor Neurons to Excitotoxic Insults through Activation of TrkB. J. Neurochem. 2003, 84, 1421–1430. [Google Scholar] [CrossRef] [Green Version]
- Mojsilovic-Petrovic, J.; Arneja, A.; Kalb, R.G. Enprofylline Protects Motor Neurons from in Vitro Excitotoxic Challenge. Neurodegener. Dis. 2005, 2, 160–165. [Google Scholar] [CrossRef]
- Yanpallewar, S.; Fulgenzi, G.; Tomassoni-Ardori, F.; Barrick, C.; Tessarollo, L. Delayed Onset of Inherited ALS by Deletion of the BDNF Receptor TrkB.T1 Is Non-Cell Autonomous. Exp. Neurol. 2021, 337, 113576. [Google Scholar] [CrossRef]
- Arango-Lievano, M.; Agustin, A.; Jeanneteau, F. Probdnf Biology and Emerging Roles in the CNS: The Unexpected Journey of Proneurotrophins. In Brain-Derived Neurotrophic Factor (BDNF): Therapeutic Approaches, Role in Neuronal Development and Effects on Cognitive Health; Nova Biomedical: Waltham, MA, USA, 2015; ISBN 978-1-63483-761-3. [Google Scholar]




| SERUM | ALS n = 75 | AD n = 19 | ID n = 20 (12 + 8) * | CTR n = 49 |
|---|---|---|---|---|
| Mean age | 64.1 ± 10.1 | 66.8 ± 7.4 | 44.7 ± 17.8 ** | 60.4 ± 10.8 |
| (range) | 37–87 y | 52–77 y | 19–85 y | 42–89 y |
| Sex | M 36 (48.0%) | M 6 (31.6%) | M 11 (55.0%) | M 23 (47%) |
| (male/female) | F 39 (52.0%) | F 13 (68.4%) | F 9 (45.0%) | F 26 (53%) |
| CSF | ALS n = 34 | AD n = 10 | ID n = 19 | CTR n = 11 |
| Mean age | 63.3 ± 8.8 | 66.9 ± 8.8 | 44.3 ± 21.5 | 60.3 ± 18.7 |
| (range) | 39–80 y | 52–77 y | 19–85 y | 39–80 y |
| Sex | M 17 (50.0%) | M 3 (30.0%) | M 10 (52.6%) | M 4 (36.4%) |
| (male/female) | F 17 (50.0%) | F 7 (70.0%) | F 9 (47.4%) | F 7 (63.6%) |
| ALS Patients | n = 75 |
|---|---|
| Site of onset (spinal/bulbar) | S 60 (80%)/B 15 (20%) |
| Genetics (C9orf72+) | 12 (16.0%) |
| Median BMI | 24.82 ± 3.98 |
| (<25: 52.5%; >25: 47.5%) | |
| Onset-diagnosis period | 13.1 ± 8.9 months |
| Disease duration | 37.2 ± 21.9 months |
| BDNF/Pro-BDNF | p Value (Compared to CTR) | |
|---|---|---|
| CTR | 0.88 ± 0.134 | |
| ALS | 2.53 ± 0.384 | <0.00001 |
| AD | 4.93 ± 0.479 | <0.00001 |
| ID | 1.18 ± 0.146 | 0.02729 |
| Sex | Site of Onset | Age of Onset * | C9orf72 | BMI | Progression Rate | |
|---|---|---|---|---|---|---|
| BDNF p value | 0.889 | 0.373 | 0.306 | 0.026 | 0.976 | 0.354 |
| Pro-BDNF p value | 0.352 | 0.276 | 0.593 | 0.749 | 0.035 | 0.534 |
| Sex | Site of Onset | Age of Onset | C9orf72 Expansion | BMI | Progression Rate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | S | B | <45 y | >45 y | C9orf72+ | C9orf72− | <25 | >25 | Fast | S + I | |
| ↑B ↑P | 13.9 | 15.4 | 15.0 | 13.3 | 40.0 | 12.9 | 8.3 | 14.8 | 18.2 | 5.3 | 14.3 | 16.0 |
| ↑B ↓P | 72.2 | 56.4 | 66.7 | 53.4 | 40.0 | 65.7 | 33.3 | 70.5 | 45.4 | 63.2 | 57.1 | 68.0 |
| ↓B ↓P | 13.9 | 28.2 | 18.3 | 33.3 | 20.0 | 21.4 | 58.4 | 14.7 | 36.4 | 31.5 | 28.6 | 16.0 |
| p value | 0.275 | 0.669 | 0.462 | 0.007 | 0.452 | 0.639 | ||||||
| Sex | Site of Onset | Age of Onset ¹ | C9orf72 | BMI | Progression Rate | Survival 2 | |
|---|---|---|---|---|---|---|---|
| p value | 0.522 | 0.104 | 0.445 | 0.813 | 0.984 | 0.026 | 0.758 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riolo, G.; Ricci, C.; De Angelis, N.; Marzocchi, C.; Guerrera, G.; Borsellino, G.; Giannini, F.; Battistini, S. BDNF and Pro-BDNF in Amyotrophic Lateral Sclerosis: A New Perspective for Biomarkers of Neurodegeneration. Brain Sci. 2022, 12, 617. https://doi.org/10.3390/brainsci12050617
Riolo G, Ricci C, De Angelis N, Marzocchi C, Guerrera G, Borsellino G, Giannini F, Battistini S. BDNF and Pro-BDNF in Amyotrophic Lateral Sclerosis: A New Perspective for Biomarkers of Neurodegeneration. Brain Sciences. 2022; 12(5):617. https://doi.org/10.3390/brainsci12050617
Chicago/Turabian StyleRiolo, Giulia, Claudia Ricci, Nicoletta De Angelis, Carlotta Marzocchi, Gisella Guerrera, Giovanna Borsellino, Fabio Giannini, and Stefania Battistini. 2022. "BDNF and Pro-BDNF in Amyotrophic Lateral Sclerosis: A New Perspective for Biomarkers of Neurodegeneration" Brain Sciences 12, no. 5: 617. https://doi.org/10.3390/brainsci12050617
APA StyleRiolo, G., Ricci, C., De Angelis, N., Marzocchi, C., Guerrera, G., Borsellino, G., Giannini, F., & Battistini, S. (2022). BDNF and Pro-BDNF in Amyotrophic Lateral Sclerosis: A New Perspective for Biomarkers of Neurodegeneration. Brain Sciences, 12(5), 617. https://doi.org/10.3390/brainsci12050617