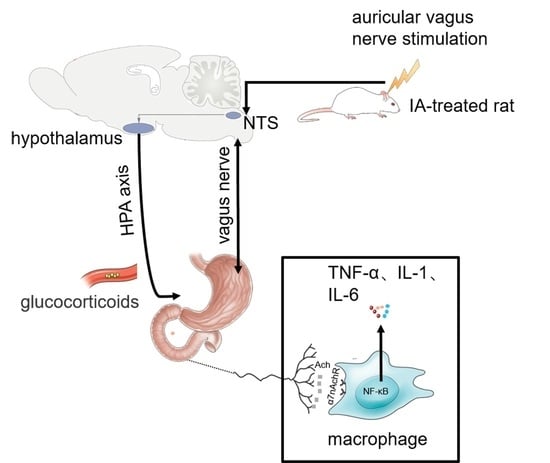
Auricular Vagus Nerve Stimulation Improves Visceral Hypersensitivity and Gastric Motility and Depression-like Behaviors via Vago-Vagal Pathway in a Rat Model of Functional Dyspepsia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Model of “FD”
2.3. Experimental Protocols
- Control group: normal rats without any IA treatment and aVNS or sham, but received balloon and wires implantation of EMG and ECG (n = 12; 6 rats were randomly picked and sacrificed for the gastric emptying test).
- IA-treated group: IA-treated rats received balloon and wires implantation of EMG and ECG but not aVNS or sham (n = 12; 6 rats were randomly chosen and sacrificed for the gastric emptying test).
- aVNS group: IA-treated rats received aVNS and balloon and wires implantation of EMG and ECG (6 IA-treated rats with daily aVNS).
- Sham-aVNS group: IA-treated rats received sham-aVNS and balloon and wires implantation of EMG and ECG (6 IA-treated rats with daily sham-aVNS).
2.4. Surgical Procedures
2.5. Auricular Vagus Nerve Stimulation
2.6. Measurements
2.6.1. Assessment of Serum Cytokines Tumor Necrosis Factor α (TNF-α), Interleukin 6 (IL-6), Interleukin 1β (IL-1β), Adreno-Cortico-Tropic-Hormone (ACTH) and Corticosterone
2.6.2. Gastric Tissue Acetylcholine (Ach)
2.6.3. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Neonatal IA Treatment-Induced Visceral Hypersensitivity, Gastric Dysmotility and Decreased OFT Scores
3.2. Effects of aVNS on Gastric Hypersensitivity, Gastric Dysmotility and Depression-Like Behaviors
3.3. aVNS Improved Vagal Activity and Gastric Tissue Level of Ach and Expression of Its Receptor
3.4. aVNS Suppressed Inflammation and Improved Impaired Mucosal Integrity
3.5. aVNS Inhibited Hyperactivation of the HPA Axis
3.6. Vagotomy Abolished the Ameliorating Effect of aVNS on Gastric Emptying, and Horizontal Motions
3.7. Vagotomy Abolished the Anti-Inflammation of aVNS
4. Discussion
4.1. The Iodoacetamide Induced FD-Like Behaviors in Rats
4.2. The Integrative Effects of aVNS for FD-Like Rats
4.3. The Ameliorating Effect of aVNS on Visceral Hypersensitivity Probably by Activating the Anti-Inflammation and Improving the Mucosal Integrity in Duodenum
4.4. aVNS Promoted the Gastric Motility Probably by Improving Vagal Activity
4.5. aVNS Alleviated Depression-Like Behaviors Probably via Downregulating the Hyperactivity HPA Axis or Brain CRF Signaling Pathway
4.6. Clinical Perspective
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Western Blotting
References
- Ford, A.C.; Mahadeva, S.; Carbone, M.F.; Lacy, B.E.; Talley, N.J. Functional dyspepsia. Lancet 2020, 396, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Palsson, O.S.; Tornblom, H.; Sperber, A.D.; Whitehead, W.E.; Simren, M. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: A cross-sectional population-based study. Lancet Gastroenterol. Hepatol. 2018, 3, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Lacy, B.E.; Andrews, C.N.; Enns, R.A.; Howden, C.W.; Vakil, N. ACG and CAG Clinical Guideline: Management of Dyspepsia. Am. J. Gastroenterol. 2017, 112, 988–1013. [Google Scholar] [CrossRef]
- Van Den Houte, K.; Carbone, F.; Tack, J. Postprandial distress syndrome: Stratification and management. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Weiser, K.T.; Kennedy, A.T.; Crowell, M.D.; Talley, N.J. Functional dyspepsia: The economic impact to patients. Aliment. Pharmacol. Ther. 2013, 38, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Hasler, W.L. Rome IV—Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- Stanghellini, V.; Chan, F.K.L.; Hasler, W.L.; Malagelada, J.R.; Suzuki, H.; Tack, J.; Talley, N.J. Gastroduodenal Disorders. Gastroenterology 2016, 150, 1380–1392. [Google Scholar] [CrossRef]
- Ford, A.C.; Luthra, P.; Tack, J.; Boeckxstaens, G.E.; Moayyedi, P.; Talley, N.J. Efficacy of psychotropic drugs in functional dyspepsia: Systematic review and meta-analysis. Gut 2017, 66, 411–420. [Google Scholar] [CrossRef]
- Frøkjaer, J.B.; Bergmann, S.; Brock, C.; Madzak, A.; Farmer, A.D.; Ellrich, J.; Drewes, A.M. Modulation of vagal tone enhances gastroduodenal motility and reduces somatic pain sensitivity. Neurogastroenterol. Motil. 2016, 28, 592–598. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, F.; Lu, D.; Rong, P.; Cheng, J.; Li, M.; Gong, Y.; Sun, C.; Wei, W.; Lin, L. Transcutaneous auricular vagal nerve stimulation improves functional dyspepsia by enhancing vagal efferent activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G700–G711. [Google Scholar] [CrossRef]
- Zhou, J.; Li, S.; Wang, Y.; Lei, Y.; Foreman, R.D.; Yin, J.; Chen, J.D. Effects and mechanisms of auricular electroacupuncture on gastric hypersensitivity in a rodent model of functional dyspepsia. PLoS ONE 2017, 12, e174568. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Ford, A.C. Functional Dyspepsia. N. Engl. J. Med. 2015, 373, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Talley, N.J. Functional dyspepsia and duodenal eosinophilia: A new model. J. Dig. Dis. 2017, 18, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J. Functional dyspepsia: New insights into pathogenesis and therapy. Korean J. Intern. Med. 2016, 31, 444–456. [Google Scholar] [CrossRef]
- Liu, L.S.; Winston, J.H.; Shenoy, M.M.; Song, G.Q.; Chen, J.D.Z.; Pasricha, P.J. A Rat Model of Chronic Gastric Sensorimotor Dysfunction Resulting from Transient Neonatal Gastric Irritation. Gastroenterology 2008, 134, 2070–2079. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Liu, Y.; Ye, F.; Yin, J.; Foreman, R.D.; Chen, J.D.Z. Electroacupuncture via chronically implanted electrodes improves gastric dysmotility mediated by autonomic-cholinergic mechanisms in a rodent model of functional dyspepsia. Neurogastroenterol. Motil. 2018, 30, e13381. [Google Scholar] [CrossRef]
- Dong, H.; Qin, Y.Q.; Sun, Y.C.; Yao, H.J.; Cheng, X.K.; Yu, Y.; Lu, S.S. Electroacupuncture Ameliorates Depressive-Like Behaviors in Poststroke Rats via Activating the tPA/BDNF/TrkB Pathway. Neuropsychiatr. Dis. Treat. 2021, 17, 1057–1067. [Google Scholar] [CrossRef]
- Thomas, N.; Gurvich, C.; Kulkarni, J. Borderline personality disorder, trauma, and the hypothalamus-pituitary-adrenal axis. Neuropsychiatr. Dis. Treat. 2019, 15, 2601–2612. [Google Scholar] [CrossRef]
- Liu, S.; Hagiwara, S.; Bhargava, A. Early-life adversity, epigenetics, and visceral hypersensitivity. Neurogastroenterol. Motil. 2017, 29, e13170. [Google Scholar] [CrossRef]
- Nishio, H.; Hayashi, Y.; Terashima, S.; Takeuchi, K. Role of endogenous nitric oxide in mucosal defense of inflamed rat stomach following iodoacetamide treatment. Life Sci. 2006, 79, 1523–1530. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Sapolsky, R.; Liao, M.; Mehta, K.; Bhargava, A.; Pasricha, P.J. Transient gastric irritation in the neonatal rats leads to changes in hypothalamic CRF expression, depression- and anxiety-like behavior as adults. PLoS ONE 2011, 6, e19498. [Google Scholar] [CrossRef] [PubMed]
- Masuy, I.; Van Oudenhove, L.; Tack, J. Review article: Treatment options for functional dyspepsia. Aliment. Pharmacol. Ther. 2019, 49, 1134–1172. [Google Scholar] [CrossRef] [PubMed]
- Gottfried-Blackmore, A.; Adler, E.P.; Fernandez-Becker, N.; Clarke, J.; Habtezion, A.; Nguyen, L. Open-label pilot study: Non-invasive vagal nerve stimulation improves symptoms and gastric emptying in patients with idiopathic gastroparesis. Neurogastroenterol. Motil. 2020, 32, e13769. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Tracey, K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef]
- Peuker, E.T.; Filler, T.J. The nerve supply of the human auricle. Clin. Anat. 2002, 15, 35–37. [Google Scholar] [CrossRef]
- He, W.; Jing, X.H.; Zhu, B.; Zhu, X.L.; Li, L.; Bai, W.Z.; Ben, H. The auriculo-vagal afferent pathway and its role in seizure suppression in rats. Bmc Neurosci. 2013, 14, 85. [Google Scholar] [CrossRef]
- Rahman, S.U.; Ali, T.; Hao, Q.; He, K.; Li, W.; Ullah, N.; Zhang, Z.; Jiang, Y.; Li, S. Xanthohumol Attenuates Lipopolysaccharide-Induced Depressive Like Behavior in Mice: Involvement of NF-kappaB/Nrf2 Signaling Pathways. Neurochem. Res. 2021, 46, 3135–3148. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Liu, C.; Wu, X.; Zhang, Y. Antidepressant mechanism of catalpol: Involvement of the PI3K/Akt/Nrf2/HO-1 signaling pathway in rat hippocampus. Eur. J. Pharmacol. 2021, 909, 174396. [Google Scholar] [CrossRef]
- Khakpai, F.; Ramezanikhah, M.; Valizadegan, F.; Zarrindast, M.R. Synergistic effect between imipramine and citicoline upon induction of analgesic and antidepressant effects in mice. Neurosci. Lett. 2021, 760, 136095. [Google Scholar] [CrossRef]
- Rong, P.; Liu, J.; Wang, L.; Liu, R.; Fang, J.; Zhao, J.; Wang, H.; Vangel, M.; Sun, S.; Ben, H.; et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study. J. Affect. Disord. 2016, 195, 172–179. [Google Scholar] [CrossRef]
- Kao, K.-L.; Sung, F.-C.; Huang, H.-C.; Lin, C.-J.; Chen, S.-C.; Lin, C.-L.; Huang, Y.-P.; Wu, S.-I.; Chen, Y.-S.; Stewart, R. Functional dyspepsia in depression: A population-based cohort study. Eur. J. Clin. Investig. 2021, 51, e13506. [Google Scholar] [CrossRef] [PubMed]
- Koloski, N.A.; Jones, M.; Kalantar, J.; Weltman, M.; Zaguirre, J.; Talley, N.J. The brain--gut pathway in functional gastrointestinal disorders is bidirectional: A 12-year prospective population-based study. Gut 2012, 61, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Gao, G.; Guo, X.; Zhang, Y.; Zhang, Z.; Wang, Y.; Zhang, J.; Wang, J.; Li, L.; et al. Transcutaneous Auricular Vagus Nerve Stimulation at 20 Hz Improves Depression-Like Behaviors and Down-Regulates the Hyperactivity of HPA Axis in Chronic Unpredictable Mild Stress Model Rats. Front. Neurosci. 2020, 14, 680. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Vagus nerve stimulation: A new promising therapeutic tool in inflammatory bowel disease. J. Intern. Med. 2017, 282, 46–63. [Google Scholar] [CrossRef]
- Wang, M.; Dong, W.; Wang, R.; Xu, X.; Wu, Y.; Sun, G.; Sun, X. Gastrodiae Rhizoma Water Extract Ameliorates Hypothalamic-Pituitary-Adrenal Axis Hyperactivity and Inflammation Induced by Chronic Unpredictable Mild Stress in Rats. Biomed. Res. Int. 2020, 2020, 8374614. [Google Scholar] [CrossRef]
- Niemegeers, P.; De Boer, P.; Dumont, G.; Van Den Eede, F.; Fransen, E.; Claes, S.J.; Morrens, M.; Sabbe, B.G.C. Differential Effects of Inflammatory and Psychosocial Stress on Mood, Hypothalamic-Pituitary-Adrenal Axis, and Inflammation in Remitted Depression. Neuropsychobiology 2016, 74, 150–158. [Google Scholar] [CrossRef]
- Rodiño-Janeiro, B.K.; Alonso-Cotoner, C.; Pigrau, M.; Lobo, B.; Vicario, M.; Santos, J. Role of Corticotropin-releasing Factor in Gastrointestinal Permeability. J. Neurogastroenterol. 2015, 21, 33–50. [Google Scholar] [CrossRef]
- Tache, Y.; Larauche, M.; Yuan, P.Q.; Million, M. Brain and Gut CRF Signaling: Biological Actions and Role in the Gastrointestinal Tract. Curr. Mol. Pharmacol. 2018, 11, 51–71. [Google Scholar] [CrossRef]
- Shi, X.; Hu, Y.; Zhang, B.; Li, W.; Chen, J.D.; Liu, F. Ameliorating effects and mechanisms of transcutaneous auricular vagal nerve stimulation on abdominal pain and constipation. JCI Insight 2021, 6, e150052. [Google Scholar] [CrossRef]
- Krasaelap, A.; Sood, M.R.; Li, B.; Unteutsch, R.; Yan, K.; Nugent, M.; Simpson, P.; Kovacic, K. Efficacy of Auricular Neurostimulation in Adolescents with Irritable Bowel Syndrome in a Randomized, Double-Blind Trial. Clin. Gastroenterol. Hepatol. 2020, 18, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Lu, X.; Zhang, S.; Zhu, C. Sini-San Regulates the NO-cGMP-PKG Pathway in the Spinal Dorsal Horn in a Modified Rat Model of Functional Dyspepsia. Evid. Based Compl. Alt. 2020, 2020, 3575231. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S. The effect of auricular electroacupuncture on the neuronal activity of the thalamic and hypothalamic neurons of the rat. Acupunct. Electrother. Res. 1986, 11, 15–23. [Google Scholar] [CrossRef]
- Ay, I.; Napadow, V.; Ay, H. Electrical stimulation of the vagus nerve dermatome in the external ear is protective in rat cerebral ischemia. Brain Stimul. 2015, 8, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, S.; Zhang, J.; Zhou, J.; Ran, H.; Tang, Y.; Chen, J.; Wang, Y. Effects and mechanisms of auricular electroacupuncture on visceral pain induced by colorectal distension in conscious rats. Acupunct. Med. 2014, 32, 472–477. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Rong, P.; Yang, Y.; Fang, J.; Wang, J.; Wang, Y.; Zhang, J.; Zhang, S.; Zhang, Z.; Chen, J.D.Z.; et al. Auricular Vagus Nerve Stimulation Improves Visceral Hypersensitivity and Gastric Motility and Depression-like Behaviors via Vago-Vagal Pathway in a Rat Model of Functional Dyspepsia. Brain Sci. 2023, 13, 253. https://doi.org/10.3390/brainsci13020253
Hou L, Rong P, Yang Y, Fang J, Wang J, Wang Y, Zhang J, Zhang S, Zhang Z, Chen JDZ, et al. Auricular Vagus Nerve Stimulation Improves Visceral Hypersensitivity and Gastric Motility and Depression-like Behaviors via Vago-Vagal Pathway in a Rat Model of Functional Dyspepsia. Brain Sciences. 2023; 13(2):253. https://doi.org/10.3390/brainsci13020253
Chicago/Turabian StyleHou, Liwei, Peijing Rong, Yang Yang, Jiliang Fang, Junying Wang, Yu Wang, Jinling Zhang, Shuai Zhang, Zixuan Zhang, Jiande D. Z. Chen, and et al. 2023. "Auricular Vagus Nerve Stimulation Improves Visceral Hypersensitivity and Gastric Motility and Depression-like Behaviors via Vago-Vagal Pathway in a Rat Model of Functional Dyspepsia" Brain Sciences 13, no. 2: 253. https://doi.org/10.3390/brainsci13020253
APA StyleHou, L., Rong, P., Yang, Y., Fang, J., Wang, J., Wang, Y., Zhang, J., Zhang, S., Zhang, Z., Chen, J. D. Z., & Wei, W. (2023). Auricular Vagus Nerve Stimulation Improves Visceral Hypersensitivity and Gastric Motility and Depression-like Behaviors via Vago-Vagal Pathway in a Rat Model of Functional Dyspepsia. Brain Sciences, 13(2), 253. https://doi.org/10.3390/brainsci13020253