Stem Cell Therapy in Diabetic Polyneuropathy: Recent Advancements and Future Directions
Abstract
:1. Introduction
2. Clinical Manifestations of DPN
3. Etiology of DPN
4. Current Management of the DPN
4.1. Prevention
4.2. Glucose Control
4.3. Pharmacological Approach
4.4. Angiogenic and Neurotrophic Factor Therapy
5. Stem Cell Therapy in DPN
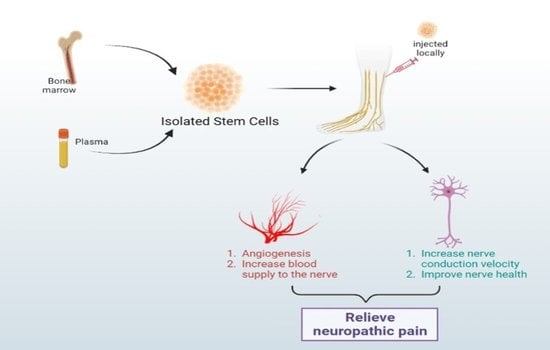
5.1. Bone Marrow Mononuclear Cell Therapy
5.2. Pluripotent Stem Cell Therapy
5.3. Endothelial Progenitor Cells (EPCs) Therapy
5.4. Mesenchymal Stromal Cells Therapy
5.5. Dental Pulp Stem Cell Therapy
5.6. Embryonic Stem Cell Therapy
6. Challenges in Cell Therapy
7. The Route of Transplantation
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Diabetes-PAHO/WHO|Pan American Health Organization, Bulletin 2022. Available online: https://www.paho.org/en/topics/diabetes (accessed on 16 December 2022).
- Singh, A.; Choubey, M.; Bora, P.; Krishna, A. Adiponectin and Chemerin: Contrary Adipokines in Regulating Reproduction and Metabolic Disorders. Reprod. Sci. 2018, 25, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Choubey, M.; Patel, S.; Singer, H.A.; Ozcan, L. Adipocyte CAMK2 deficiency improves obesity-associated glucose intolerance. Mol. Metab. 2021, 53, 101300. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L. Epidemiology and Classification of Diabetic Neuropathy-UpToDate. 2018. Available online: https://www.uptodate.com/contents/epidemiology-and-classification-of-diabetic-neuropathy (accessed on 16 December 2022).
- Dyck, P.J.; Litchy, W.J.; Lehman, K.A.; Hokanson, J.L.; Low, P.A.; O’Brien, P.C. Variables influencing neuropathic endpoints: The Rochester Diabetic Neuropathy Study of Healthy Subjects. Neurology 1995, 45, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Dyck, P.J.; Kratz, K.M.; Karnes, J.L.; Litchy, W.J.; Klein, R.; Pach, J.M.; Wilson, D.M.; O’Brien, P.C.; Melton, L.J. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The Rochester Diabetic Neuropathy Study. Neurology 1993, 43, 817–824. [Google Scholar] [CrossRef]
- Edwards, J.L.; Vincent, A.M.; Cheng, H.T.; Feldman, E.L. Diabetic neuropathy: Mechanisms to management. Pharmacol. Ther. 2008, 120, 1–34. [Google Scholar]
- Boulton, A.J.; Kirsner, R.S.; Vileikyte, L. Neuropathic Diabetic Foot Ulcers. New Engl. J. Med. 2004, 351, 48–55. [Google Scholar] [CrossRef]
- Boulton, A.J.; Vinik, A.I.; Arezzo, J.C.; Bril, V.; Feldman, E.L.; Freeman, R.; Ziegler, D. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care 2005, 28, 956–962. [Google Scholar] [CrossRef]
- Tesfaye, S.; Boulton, A.J.M.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic Neuropathies: Update on Definitions, Diagnostic Criteria, Estimation of Severity, and Treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Martin, C.L.; Albers, J.; Herman, W.H.; Cleary, P.; Waberski, B.; Greene, D.A.; Stevens, M.J.; Feldman, E.L. Neuropathy Among the Diabetes Control and Complications Trial Cohort 8 Years After Trial Completion. Diabetes Care 2006, 29, 340–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J. Pediatr. 1994, 125, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Stracke, H.; Gaus, W.; Achenbach, U.; Federlin, K.; Bretzel, R.G. Benfotiamine in diabetic polyneuropathy (BENDIP): Results of a randomised, double blind, placebo-controlled clinical study. Exp. Clin. Endocrinol. Diabetes Off. J. 2008, 116, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.D.; Alviar, M.J.M.; Dans, A.L.; Bautista-Velez GG, P.; Villaruz-Sulit MV, C.; Tan, J.J.; Roxas, A.A. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst. Rev. 2008, 16, Cd004573. [Google Scholar] [CrossRef]
- Kato, N.; Nemoto, K.; Nakanishi, K.; Morishita, R.; Kaneda, Y.; Uenoyama, M.; Fujikawa, K. Nonviral gene transfer of human hepatocyte growth factor improves streptozotocin-induced diabetic neuropathy in rats. Diabetes 2005, 54, 846–854. [Google Scholar] [CrossRef]
- Mizukami, H.; Yagihashi, S. Exploring a new therapy for diabetic polyneuropathy—The application of stem cell transplantation. Front. Endocrinol. (Lausanne) 2014, 5, 45. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Ahmadi, H.; Ghasrodashti, Z.P.; Tanideh, N.; Shahriarirad, R.; Erfani, A.; Ashkani-Esfahani, S. Therapeutic effects of stem cells in different body systems, a novel method that is yet to gain trust: A comprehensive review. Bosn. J. Basic Med. Sci. 2021, 21, 672. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Zhang, Z.; Qian, G.S. Mesenchymal stem cells to treat diabetic neuropathy: A long and strenuous way from bench to the clinic. Cell Death Discov. 2016, 2, 16055. [Google Scholar] [CrossRef]
- Jin, K.; Zhu, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Greenberg, D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11946–11950. [Google Scholar] [CrossRef]
- Donovan, M.H.; Yazdani, U.; Norris, R.D.; Games, D.; German, D.C.; Eisch, A.J. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J. Comp. Neurol. 2006, 495, 70–83. [Google Scholar] [CrossRef]
- López-Toledano, M.A.; Shelanski, M.L. Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind). J. Alzheimer’S Dis. JAD 2007, 12, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Blurton-Jones, M.; Kitazawa, M.; Martinez-Coria, H.; Castello, N.A.; Müller, F.J.; Loring, J.F.; LaFerla, F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13594–13599. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Winn, S.R.; Liu, Y.T.; Mufson, E.J.; Sladek, J.R.; Hammang, J.P.; E Baetge, E.; Emerich, D.F. The aged monkey basal forebrain: Rescue and sprouting of axotomized basal forebrain neurons after grafts of encapsulated cells secreting human nerve growth factor. Proc. Natl. Acad. Sci. USA 1994, 91, 10898–10902. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yang, Y.H.; Bae, D.K.; Lee, S.H.; Yang, G.; Kyung, J.; Kim, Y.B. Improvement of cognitive function and physical activity of aging mice by human neural stem cells over-expressing choline acetyltransferase. Neurobiol. Aging 2013, 34, 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-S.; Jung, K.; Kim, I.-S.; Lee, H.; Kim, M.; Yun, S.; Hwang, K.; Shin, J.E.; Park, K.I. Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol. Neurodegener. 2015, 10, 38. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, H.H.; Wang, Y.; Gu, G.J.; Zhang, W.; Xia, R. Neural stem cell transplantation decreases neuroinflammation in a transgenic mouse model of Alzheimer’s disease. J. Neurochem. 2016, 136, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Lilja, A.M.; Malmsten, L.; Röjdner, J.; Voytenko, L.; Verkhratsky, A.; Ögren, S.O.; Marutle, A. Neural Stem Cell Transplant-Induced Effect on Neurogenesis and Cognition in Alzheimer Tg2576 Mice Is Inhibited by Concomitant Treatment with Amyloid-Lowering or Cholinergic α7 Nicotinic Receptor Drugs. Neural Plast. 2015, 2015, 370432. [Google Scholar] [CrossRef]
- Xuan, A.G.; Luo, M.; Ji, W.D.; Long, D.H. Effects of engrafted neural stem cells in Alzheimer’s disease rats. Neurosci. Lett. 2009, 450, 167–171. [Google Scholar] [CrossRef]
- Bissonnette, C.J.; Lyass, L.; Bhattacharyya, B.J.; Belmadani, A.; Miller, R.J.; Kessler, J.A. The controlled generation of functional basal forebrain cholinergic neurons from human embryonic stem cells. Stem Cells 2011, 29, 802–811. [Google Scholar] [CrossRef]
- Park, D.; Yang, G.; Bae, D.K.; Lee, S.H.; Yang, Y.H.; Kyung, J.; Kim, Y.B. Human adipose tissue-derived mesenchymal stem cells improve cognitive function and physical activity in ageing mice. J. Neurosci. Res. 2013, 91, 660–670. [Google Scholar] [CrossRef]
- Zilka, N.; Zilkova, M.; Kazmerova, Z.; Sarissky, M.; Cigankova, V.; Novak, M. Mesenchymal stem cells rescue the Alzheimer’s disease cell model from cell death induced by misfolded truncated tau. Neuroscience 2011, 193, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.K.; Lee, H.; Carter, J.E.; Chang, J.W.; Oh, W.; Bae, J.S. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol. Aging 2012, 33, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, G.M.; Gowing, G.; Svendsen, S.; Svendsen, C.N. The past, present and future of stem cell clinical trials for ALS. Exp. Neurol. 2014, 262, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, L.; Gelati, M.; Profico, D.C.; Sgaravizzi, G.; Projetti Pensi, M.; Muzi, G.; Vescovi, A.L. Human neural stem cell transplantation in ALS: Initial results from a phase I trial. J. Transl. Med. 2015, 13, 17. [Google Scholar] [CrossRef]
- Human neural stem cell transplants improve motor function in a rat model of Huntington’s disease-McBride-2004. J. Comp. Neurol. 2004, 475, 211–219. [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Park, J.E.; Lee, K.; Kang, L.; Kim, S.U.; Kim, M. Intravenous administration of human neural stem cells induces functional recovery in Huntington’s disease rat model. Neurosci. Res. 2005, 52, 243–249. [Google Scholar] [CrossRef]
- Vazey, E.M.; Chen, K.; Hughes, S.M.; Connor, B. Transplanted adult neural progenitor cells survive, differentiate and reduce motor function impairment in a rodent model of Huntington’s disease. Exp. Neurol. 2006, 199, 384–396. [Google Scholar] [CrossRef]
- Bachoud-Lévi, A.C.; Rémy, P.; Nǵuyen, J.P.; Brugières, P.; Lefaucheur, J.P.; Bourdet, C.; Peschanski, M. Motor and cognitive improvements in patients with Huntington’s disease after neural transplantation. Lancet 2000, 356, 1975–1979. [Google Scholar] [CrossRef]
- Bachoud-Lévi, A.C.; Gaura, V.; Brugières, P.; Lefaucheur, J.P.; Boissé, M.F.; Maison, P.; Peschanski, M. Effect of fetal neural transplants in patients with Huntington’s disease 6 years after surgery: A long-term follow-up study. Lancet Neurol. 2006, 5, 303–309. [Google Scholar] [CrossRef]
- Carstens, M.H.; Gómez, A.; Cortés, R.; Turner, E.; Pérez, C.; Ocon, M.; Correa, D. Non-reconstructable peripheral vascular disease of the lower extremity in ten patients treated with adipose-derived stromal vascular fraction cells. Stem Cell Res. 2017, 18, 14–21. [Google Scholar] [CrossRef]
- Bang, O.Y.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005, 57, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hong, J.M.; Moon, G.J.; Lee, P.H.; Ahn, Y.H.; Bang, O.Y. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010, 28, 1099–1106. [Google Scholar] [CrossRef]
- Haas, S.; Weidner, N.; Winkler, J. Adult stem cell therapy in stroke. Curr. Opin. Neurol. 2005, 18, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Einstein, O.; Karussis, D.; Grigoriadis, N.; Mizrachi-Kol, R.; Reinhartz, E.; Abramsky, O.; Ben-Hur, T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol. Cell. Neurosci. 2003, 24, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, S.; Quattrini, A.; Brambilla, E.; Gritti, A.; Salani, G.; Dina, G.; Martino, G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature 2003, 422, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.; Ben-Hur, T.; Miller, B.R.; Mizrachi-Kol, R.; Einstein, O.; Reinhartz, E.; Frank, J.A. MR microscopy of magnetically labeled neurospheres transplanted into the Lewis EAE rat brain. Magn. Reson. Med. 2003, 50, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Mothe, A.J.; Tator, C.H. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int. J. Dev. Neurosci. 2013, 31, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.W.; Roskams, A.J. Olfactory ensheathing cell transplantation following spinal cord injury: Hype or hope? Exp. Neurol. 2008, 209, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G.; Sabin, T.D. Diabetic truncal polyneuropathy. Arch. Neurol. 1981, 38, 46–47. [Google Scholar] [CrossRef]
- Apfel, S.C. Neurotrophic factors in the therapy of diabetic neuropathy. Am. J. Med. 1999, 107, 34–42. [Google Scholar] [CrossRef]
- Jensen, T.S.; Finnerup, N.B. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 2014, 13, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.M.; Mclean, L.L.; Backus, C.; Feldman, E.L. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005, 19, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, D.; Singh, B.K.; Akhtar, M. Diabetic neuropathy: Therapies on the horizon. J. Pharm. Pharmacol. 2009, 61, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Nave, K.A.; Jensen, T.S.; Bennett, D.L. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 2017, 93, 1296–1313. [Google Scholar] [CrossRef] [PubMed]
- Jack, M.; Wright, D. Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy. Transl. Res. 2012, 159, 355–365. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Li, F.; Abatan, O.I.; Forsell, M.A.; Komjáti, K.; Pacher, P.; Szabó, C.; Stevens, M.J. Role of Poly(ADP-Ribose) Polymerase Activation in Diabetic Neuropathy. Diabetes 2004, 53, 711–720. [Google Scholar] [CrossRef]
- Vincent, A.M.; Callaghan, B.C.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Cellular mechanisms as therapeutic targets. Nat. Rev. Neurol. 2011, 7, 573–583. [Google Scholar] [CrossRef]
- Zacchigna, S.; Lambrechts, D.; Carmeliet, P. Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 169–181. [Google Scholar] [CrossRef]
- Pittenger, G.; Vinik, A. Nerve Growth Factor and Diabetic Neuropathy. Exp. Diabesity Res. 2003, 4, 271–285. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2010, 2, 18–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Whittemore, R.; He, G.-P. The relationship between diabetes self-management and metabolic control in youth with type 1 diabetes: An integrative review. J. Adv. Nurs. 2011, 67, 2294–2310. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Feldman, E.L. Insulin resistance in the nervous system. Trends Endocrinol. Metab. 2012, 23, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, E.D.; Weigert, C. Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney Int. 2000, 58, S13–S18. [Google Scholar] [CrossRef]
- Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann.Neurol. 1995, 38, 869–880. [CrossRef]
- Linn, T.; Ortac, K.; Laube, H.; Federlin, K. Intensive therapy in adult insulin-dependent diabetes mellitus is associated with improved insulin sensitivity and reserve: A randomized, controlled, prospective study over 5 years in newly diagnosed patients. Metabolism 1996, 45, 1508–1513. [Google Scholar] [CrossRef]
- Ismail-Beigi, F.; Craven, T.; Banerji, M.A.; Basile, J.; Calles, J.; Cohen, R.M.; Cuddihy, R.; Cushman, W.C.; Genuth, S.; Grimm, R.H.; et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the ACCORD randomised trial. Lancet 2010, 376, 419–430. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Cheng, H.T.; Stables, C.L.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet. Neurol. 2012, 11, 521–534. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Kishikawa, H.; Araki, E.; Miyata, T.; Isami, S.; Motoyoshi, S.; Kojima, Y.; Furuyoshi, N.; Shichiri, M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: A randomized prospective 6-year study. Diabetes Res. Clin. Pr. 1995, 28, 103–117. [Google Scholar] [CrossRef]
- Ang, L.; Jaiswal, M.; Martin, C.; Pop-Busui, R. Glucose Control and Diabetic Neuropathy: Lessons from Recent Large Clinical Trials. Curr. Diabetes Rep. 2014, 14, 528. [Google Scholar] [CrossRef]
- Diabetic Nerve Damage. 2022. Available online: https://www.joslin.org/patient-care/diabetes-education/diabetes-learning-center/diabetic-nerve-damage (accessed on 16 December 2022).
- Detaille, D.; Guigas, B.; Chauvin, C.; Batandier, C.; Fontaine, E.; Wiernsperger, N.; Leverve, X. Metformin Prevents High-Glucose–Induced Endothelial Cell Death Through a Mitochondrial Permeability Transition-Dependent Process. Diabetes 2005, 54, 2179–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Mir, M.-Y.; Detaille, D.; R-Villanueva, G.; Delgado-Esteban, M.; Guigas, B.; Attia, S.; Fontaine, E.; Almeida, A.; Leverve, X. Neuroprotective Role of Antidiabetic Drug Metformin Against Apoptotic Cell Death in Primary Cortical Neurons. J. Mol. Neurosci. 2007, 34, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Sjoholm, A.; Nystrom, T. Endothelial inflammation in insulin resistance. Lancet 2005, 365, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, J.; Li, D.; Zhang, X.; Mehta, J.L. Angiotensin II regulation of collagen type I expression in cardiac fibroblasts: Modulation by PPAR-gamma ligand pioglitazone. Hypertension 2004, 44, 655–661. [Google Scholar] [CrossRef]
- Wiggin, T.D.; Kretzler, M.; Pennathur, S.; Sullivan, K.A.; Brosius, F.C.; Feldman, E.L. Rosiglitazone Treatment Reduces Diabetic Neuropathy in Streptozotocin-Treated DBA/2J Mice. Endocrinology 2008, 149, 4928–4937. [Google Scholar] [CrossRef]
- Sadosky, A.; Schaefer, C.; Mann, R.; Bergstrom, F.; Baik, R.; Parsons, B.; Nalamachu, S.; Nieshoff, E.; Stacey, B.R.; Tuchman, M.; et al. Burden of illness associated with painful diabetic peripheral neuropathy among adults seeking treatment in the US: Results from a retrospective chart review and cross-sectional survey. Diabetes Metab. Syndr. Obesity Targets Ther. 2013, 6, 79–92. [Google Scholar] [CrossRef]
- Smith, A.G.; Russell, J.; Feldman, E.L.; Goldstein, J.; Peltier, A.; Smith, S.; Hamwi, J.; Pollari, D.; Bixby, B.; Howard, J.; et al. Lifestyle Intervention for Pre-Diabetic Neuropathy. Diabetes Care 2006, 29, 1294–1299. [Google Scholar] [CrossRef]
- Oyibo, S.O.; Prasad, Y.D.M.; Jackson, N.J.; Jude, E.B.; Boulton, A.J.M. The relationship between blood glucose excursions and painful diabetic peripheral neuropathy: A pilot study. Diabet. Med. 2002, 19, 870–873. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Griebeler, M.L.; Morey-Vargas, O.L.; Brito, J.P.; Tsapas, A.; Wang, Z.; Carranza Leon, B.G.; Murad, M.H. Pharmacologic interventions for painful diabetic neuropathy: An umbrella systematic review and comparative effectiveness network meta-analysis. Ann. Intern. Med. 2014, 161, 639–649. [Google Scholar] [CrossRef]
- Freeman, R.; Durso-DeCruz, E.; Emir, B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: Findings from seven randomized, controlled trials across a range of doses. Diabetes Care 2008, 31, 1448–1454. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.A.; Straube, S.; Wiffen, P.J.; Derry, S.; McQuay, H.J. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst. Rev. 2009, 8, CD007076. [Google Scholar] [CrossRef]
- Raskin, P.; Huffman, C.; Toth, C.; Asmus, M.J.; Messig, M.; Sanchez, R.J.; Pauer, L. Pregabalin in patients with inadequately treated painful diabetic peripheral neuropathy: A randomized withdrawal trial. Clin. J. Pain 2014, 30, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Wilhelm, S.; Lledo, A.; Schacht, A.; Tölle, T.; Bouhassira, D.; Freynhagen, R. Duloxetine and pregabalin: High-dose monotherapy or their combination? The “COMBO-DN study”--a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain 2013, 154, 2616–2625. [Google Scholar] [CrossRef]
- Ziegler, D.; Duan, W.R.; An, G.; Thomas, J.W.; Nothaft, W. A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain. Pain 2015, 156, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Quilici, S.; Chancellor, J.; Löthgren, M.; Simon, D.; Said, G.; Le, T.K.; Garcia-Cebrian, A.; Monz, B. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Bril, V.; England, J.; Franklin, G.M.; Backonja, M.; Cohen, J.; Del Toro, D.; Zochodne, D. Evidence-based guideline: Treatment of painful diabetic neuropathy: Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Pm&r 2011, 3, 345–352.e21. [Google Scholar]
- Dworkin, R.H.; O’Connor, A.B.; Backonja, M.; Farrar, J.T.; Finnerup, N.B.; Jensen, T.S.; Kalso, E.A.; Loeser, J.D.; Miaskowski, C.; Nurmikko, T.J.; et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain 2007, 132, 237–251. [Google Scholar] [CrossRef]
- McQuay, H.J.; Tramér, M.; Nye, B.A.; Carroll, D.; Wiffen, P.J.; Moore, R.A. A systematic review of antidepressants in neuropathic pain. Pain 1996, 68, 217–227. [Google Scholar] [CrossRef]
- Mohiuddin, M.S.; Himeno, T.; Inoue, R.; Miura-Yura, E.; Yamada, Y.; Nakai-Shimoda, H.; Kamiya, H. Glucagon-Like Peptide-1 Receptor Agonist Protects Dorsal Root Ganglion Neurons against Oxidative Insult. J. Diabetes Res. 2019, 2019, 9426014. [Google Scholar] [CrossRef]
- Motegi, M.; Himeno, T.; Nakai-Shimoda, H.; Inoue, R.; Ozeki, N.; Hayashi, Y.; Kamiya, H. Deficiency of glucagon gene-derived peptides induces peripheral polyneuropathy in mice. Biochem. Biophys. Res. Commun. 2020, 532, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.S.; Himeno, T.; Yamada, Y.; Morishita, Y.; Kondo, M.; Tsunekawa, S.; Kamiya, H. Glucagon Prevents Cytotoxicity Induced by Methylglyoxal in a Rat Neuronal Cell Line Model. Biomolecules 2021, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2016, 40, 136–154. [Google Scholar] [CrossRef]
- Schmidt, R.E.; Dorsey, D.A.; Beaudet, L.N.; Plurad, S.B.; Parvin, C.A.; Miller, M.S. Insulin-like Growth Factor I Reverses Experimental Diabetic Autonomic Neuropathy. Am. J. Pathol. 1999, 155, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.X.; Snyder, C.K.; Pu, S.F.; Ishii, D.N. Insulin-like Growth Factors Reverse or Arrest Diabetic Neuropathy: Effects on Hyperalgesia and Impaired Nerve Regeneration in Rats. Exp. Neurol. 1996, 140, 198–205. [Google Scholar] [CrossRef]
- Mizisin, A.P.; Vu, Y.; Shuff, M.; Calcutt, N.A. Ciliary Neurotrophic Factor Improves Nerve Conduction and Ameliorates Regeneration Deficits in Diabetic Rats. Diabetes 2004, 53, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Anitha, M.; Gondha, C.; Sutliff, R.; Parsadanian, A.; Mwangi, S.; Sitaraman, S.V.; Srinivasan, S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J. Clin. Investig. 2006, 116, 344–356. [Google Scholar] [CrossRef]
- Ropper, A.H.; Gorson, K.C.; Gooch, C.L.; Weinberg, D.H.; Pieczek, A.; Ware, J.H.; Kershen, J.; Rogers, A.; Simovic, D.; Schratzberger, P.; et al. Vascular endothelial growth factor gene transfer for diabetic polyneuropathy: A randomized, double-blinded trial. Ann. Neurol. 2009, 65, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Nakae, M.; Kamiya, H.; Naruse, K.; Horio, N.; Ito, Y.; Mizubayashi, R.; Hamada, Y.; Nakashima, E.; Akiyama, N.; Kobayashi, Y.; et al. Effects of Basic Fibroblast Growth Factor on Experimental Diabetic Neuropathy in Rats. Diabetes 2006, 55, 1470–1477. [Google Scholar] [CrossRef]
- Tacken, P.J.; De Vries, I.J.M.; Torensma, R.; Figdor, C. Dendritic-cell immunotherapy: From ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 2007, 7, 790–802. [Google Scholar] [CrossRef]
- Prochazka, V.; Gumulec, J.; Chmelova, J.; Klement, P.; Klement, G.L.; Jonszta, T.; Krajca, J. Autologous bone marrow stem cell transplantation in patients with end-stage chronical critical limb ischemia and diabetic foot. Vnitr Lek 2009, 55, 173–178. [Google Scholar] [PubMed]
- Cuende, N.; Rico, L.; Herrera, C. Concise Review: Bone Marrow Mononuclear Cells for the Treatment of Ischemic Syndromes: Medicinal Product or Cell Transplantation? Stem Cells Transl. Med. 2012, 1, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Rehman, J. Redox and Metabolic Regulation of Stem/Progenitor Cells and Their Niche. Antioxidants Redox Signal. 2014, 21, 1587–1590. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.; Jones, D.R.; Scotting, P.; Sottile, V. Adult mesenchymal stem cells: Differentiation potential and therapeutic applications. J. Postgrad. Med. 2007, 53, 121–127. [Google Scholar] [PubMed]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Shou, M.; Lee, C.W.; Barr, S.; Epstein, S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004, 109, 1543–1549. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kosaki, A.; Shimizu, K.; Matsubara, H.; Mori, Y.; Masaki, H.; Toyoda, N.; Inoue-Shibata, M.; Nishikawa, M.; Iwasaka, T. Amelioration of diabetic peripheral neuropathy by implantation of hematopoietic mononuclear cells in streptozotocin-induced diabetic rats. Exp. Neurol. 2006, 199, 274–280. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.-S.; Choi, Y.J.; Kim, M.-O.; Huh, Y.H.; Kim, S.-W.; Han, J.W.; Lee, J.; Kim, S.; Houge, M.A.; et al. Bone Marrow Mononuclear Cells Have Neurovascular Tropism and Improve Diabetic Neuropathy. Stem Cells 2009, 27, 1686–1696. [Google Scholar] [CrossRef]
- Shibata, T.; Naruse, K.; Kamiya, H.; Kozakae, M.; Kondo, M.; Yasuda, Y.; Nakamura, N.; Ota, K.; Tosaki, T.; Matsuki, T.; et al. Transplantation of Bone Marrow–Derived Mesenchymal Stem Cells Improves Diabetic Polyneuropathy in Rats. Diabetes 2008, 57, 3099–3107. [Google Scholar] [CrossRef]
- Kondo, M.; Kamiya, H.; Himeno, T.; Naruse, K.; Nakashima, E.; Watarai, A.; Shibata, T.; Tosaki, T.; Kato, J.; Okawa, T.; et al. Therapeutic efficacy of bone marrow-derived mononuclear cells in diabetic polyneuropathy is impaired with aging or diabetes. J. Diabetes Investig. 2014, 6, 140–149. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Han, J.W.; Kim, J.-M.; Cho, H.-J.; Park, C.; Lee, N.; Kim, D.-W.; Yoon, Y.-S. Malignant Tumor Formation After Transplantation of Short-Term Cultured Bone Marrow Mesenchymal Stem Cells in Experimental Myocardial Infarction and Diabetic Neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Takizawa, N.; Narita, M.; Ichisaka, T.; Yamanaka, S. Promotion of direct reprogramming by transformation-deficient Myc. Proc. Natl. Acad. Sci. 2010, 107, 14152–14157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, M.; Yamaguchi, K.; Nakamura, T.; Shibukawa, R.; Kodanaka, I.; Ichisaka, T.; Kawamura, Y.; Mochizuki, H.; Goshima, N.; Yamanaka, S. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature 2011, 474, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Naruse, K.; Hamada, Y.; Nakashima, E.; Kato, K.; Mizubayashi, R.; Kamiya, H.; Yuzawa, Y.; Matsuo, S.; Murohara, T.; Matsubara, T.; et al. Therapeutic Neovascularization Using Cord Blood–Derived Endothelial Progenitor Cells for Diabetic Neuropathy. Diabetes 2005, 54, 1823–1828. [Google Scholar] [CrossRef]
- Park, T.S.; Bhutto, I.; Zimmerlin, L.; Huo, J.S.; Nagaria, P.; Miller, D.; Zambidis, E.T. Vascular Progenitors from Cord Blood-Derived iPSC Possess Augmented Capacity for Regenerating Ischemic Retinal Vasculature. Circulation 2014, 129, 359–372. [Google Scholar] [CrossRef]
- Okawa, T.; Kamiya, H.; Himeno, T.; Kato, J.; Seino, Y.; Fujiya, A.; Kondo, M.; Tsunekawa, S.; Naruse, K.; Hamada, Y.; et al. Transplantation of Neural Crest-Like Cells Derived from Induced Pluripotent Stem Cells Improves Diabetic Polyneuropathy in Mice. Cell Transplant. 2013, 22, 1767–1783. [Google Scholar] [CrossRef]
- Ohnuki, M.; Takahashi, K. Present and future challenges of induced pluripotent stem cells. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140367. [Google Scholar] [CrossRef]
- Timmermans, F.; Plum, J.; Yöder, M.C.; Ingram, D.A.; Vandekerckhove, B.; Case, J. Endothelial progenitor cells: Identity defined? J. Cell Mol. Med. 2009, 13, 87–102. [Google Scholar] [CrossRef]
- Basile, D.P.; Yoder, M.C. Circulating and tissue resident endothelial progenitor cells. J. Cell. Physiol. 2014, 229, 10–16. [Google Scholar] [CrossRef]
- Shi, Q.; Rafii, S.; Wu, M.H.; Wijelath, E.S.; Yu, C.; Ishida, A.; Fujita, Y.; Kothari, S.; Mohle, R.; Sauvage, L.R.; et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998, 92, 362–367. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Kim, M.-O.; Kim, H.; Lee, M.-Y.; Kim, S.-W.; Ii, M.; Lee, J.-U.; Lee, J.; Choi, Y.J.; Cho, H.-J.; et al. Dual Angiogenic and Neurotrophic Effects of Bone Marrow–Derived Endothelial Progenitor Cells on Diabetic Neuropathy. Circulation 2009, 119, 699–708. [Google Scholar] [CrossRef]
- Rehman, J.; Li, J.; Orschell, C.M.; March, K.L. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 2003, 107, 1164–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.-J.; Lee, N.; Lee, J.Y.; Choi, Y.J.; Ii, M.; Wecker, A.; Jeong, J.-O.; Curry, C.; Qin, G.; Yoon, Y.-S. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J. Exp. Med. 2007, 204, 3257–3269. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, T.J., IV; Wamhoff, B.R.; Owens, G.K.; Skalak, T.C. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ. Res. 2005, 97, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Ziegelhoeffer, T.; Fernandez, B.; Kostin, S.; Heil, M.; Voswinckel, R.; Helisch, A.; Schaper, W. Bone Marrow-Derived Cells Do Not Incorporate Into the Adult Growing Vasculature. Circ. Res. 2004, 94, 230–238. [Google Scholar] [CrossRef]
- Porada, C.D.; Zanjani, E.D.; Almeida-Porad, G. Adult mesenchymal stem cells: A pluripotent population with multiple applications. Curr. Stem Cell Res. Ther. 2006, 1, 365–369. [Google Scholar] [CrossRef]
- Friedman, R.; Betancur, M.; Boissel, L.; Tuncer, H.; Cetrulo, C.; Klingemann, H. Umbilical Cord Mesenchymal Stem Cells: Adjuvants for Human Cell Transplantation. Biol. Blood Marrow Transplant. 2007, 13, 1477–1486. [Google Scholar] [CrossRef]
- Zhang, W.; Walboomers, X.F.; Van Kuppevelt, T.H.; Daamen, W.F.; Van Damme, P.A.; Bian, Z.; A Jansen, J. In vivo evaluation of human dental pulp stem cells differentiated towards multiple lineages. J. Tissue Eng. Regen. Med. 2008, 2, 117–125. [Google Scholar] [CrossRef]
- Pereira, R.F.; Halford, K.W.; O’Hara, M.D.; Leeper, D.B.; Sokolov, B.P.; Pollard, M.D.; Bagasra, O.; Prockop, D.J. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc. Natl. Acad. Sci. USA 1995, 92, 4857–4861. [Google Scholar] [CrossRef]
- Poloni, A.; Maurizi, G.; Babini, L.; Serrani, F.; Berardinelli, E.; Mancini, S.; Costantini, B.; Discepoli, G.; Leoni, P. Human Mesenchymal Stem Cells from Chorionic Villi and Amniotic Fluid are not Susceptible to Transformation after Extensive in Vitro Expansion. Cell Transplant. 2011, 20, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E.; et al. No Identical “Mesenchymal Stem Cells” at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Rep. 2016, 6, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P.G.; Riminucci, M.; et al. Self-Renewing Osteoprogenitors in Bone Marrow Sinusoids Can Organize a Hematopoietic Microenvironment. Cell 2007, 131, 324–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.J.; Lee, P.H.; Bang, O.Y.; Lee, G.; Ahn, Y.H. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson’s disease. J. Neurochem. 2008, 107, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Quertainmont, R.; Cantinieaux, D.; Botman, O.; Sid, S.; Schoenen, J.; Franzen, R. Mesenchymal Stem Cell Graft Improves Recovery after Spinal Cord Injury in Adult Rats through Neurotrophic and Pro-Angiogenic Actions. PLoS ONE 2012, 7, e39500. [Google Scholar] [CrossRef] [PubMed]
- Siniscalco, D.; Giordano, C.; Galderisi, U.; Luongo, L.; Alessio, N.; Di Bernardo, G.; de Novellis, V.; Rossi, F.; Maione, S. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell. Mol. Life Sci. 2009, 67, 655–669. [Google Scholar] [CrossRef]
- Ezquer, F.E.; Ezquer, M.E.; Parrau, D.B.; Carpio, D.; Yañez, A.J.; Conget, P.A. Systemic Administration of Multipotent Mesenchymal Stromal Cells Reverts Hyperglycemia and Prevents Nephropathy in Type 1 Diabetic Mice. Biol. Blood Marrow Transplant. 2008, 14, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Omi, M.; Hata, M.; Nakamura, N.; Miyabe, M.; Ozawa, S.; Nukada, H.; Naruse, K. Transplantation of dental pulp stem cells improves long-term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Res. Ther. 2017, 8, 279. [Google Scholar] [CrossRef]
- D’Aquino, R.; Graziano, A.; Sampaolesi, M.; Laino, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007, 14, 1162–1171. [Google Scholar] [CrossRef]
- Munson, J.B.; Shelton, D.L.; McMahon, S.B. Adult mammalian sensory and motor neurons: Roles of endogenous neurotrophins and rescue by exogenous neurotrophins after axotomy. J. Neurosci. 1997, 17, 470–476. [Google Scholar] [CrossRef]
- Seitz, M.; Grosheva, M.; Skouras, E.; Angelova, S.K.; Ankerne, J.; Jungnickel, J.; Angelov, D.N. Poor functional recovery and muscle polyinnervation after facial nerve injury in fibroblast growth factor-2-/- mice can be improved by manual stimulation of denervated vibrissal muscles. Neuroscience 2011, 182, 241–247. [Google Scholar] [CrossRef]
- Fairbairn, N.G. Augmenting peripheral nerve regeneration using stem cells: A review of current opinion. World J. Stem Cells 2015, 7, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Pomp, O.; Brokhman, I.; Ben-Dor, I.; Reubinoff, B.; Goldstein, R.S. Generation of Peripheral Sensory and Sympathetic Neurons and Neural Crest Cells from Human Embryonic Stem Cells. Stem Cells 2005, 23, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kim, H.; Elkabetz, Y.; Al Shamy, G.; Panagiotakos, G.; Barberi, T.; Tabar, V.; Studer, L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat. Biotechnol. 2007, 25, 1468–1475. [Google Scholar] [CrossRef]
- Ziegler, L.; Grigoryan, S.; Yang, I.H.; Thakor, N.; Goldstein, R.S. Efficient Generation of Schwann Cells from Human Embryonic Stem Cell-Derived Neurospheres. Stem Cell Rev. Rep. 2010, 7, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, B.; Miura, T.; Brandenberger, R.; Mejido, J.; Luo, Y.; Yang, A.X.; Joshi, B.H.; Ginis, I.; Thies, R.S.; Amit, M.; et al. Gene expression in human embryonic stem cell lines: Unique molecular signature. Blood 2004, 103, 2956–2964. [Google Scholar] [CrossRef]
- Nakazawa, T.; Tamai, M.; Mori, N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Investig. Opthalmology Vis. Sci. 2002, 43, 3319–3326. [Google Scholar]
- Jones, I.; Novikova, L.N.; Novikov, L.N.; Renardy, M.; Ullrich, A.; Wiberg, M.; Carlsson, L.; Kingham, P.J. Regenerative effects of human embryonic stem cell-derived neural crest cells for treatment of peripheral nerve injury. J. Tissue Eng. Regen. Med. 2018, 12, e2099–e2109. [Google Scholar] [CrossRef]
- Annas, G.J.; Caplan, A.; Elias, S. Stem cell politics, ethics and medical progress. Nat. Med. 1999, 5, 1339–1341. [Google Scholar] [CrossRef]
- de Wert, G.; Mummery, C. Human embryonic stem cells: Research, ethics and policy. Hum. Reprod. 2003, 18, 672–682. [Google Scholar] [CrossRef]
- Blum, B.; Benvenisty, N. The Tumorigenicity of Human Embryonic Stem Cells. Adv. Cancer Res. 2008, 100, 133–158. [Google Scholar]
- Tolar, J.; Nauta, A.J.; Osborn, M.J.; Mortari, A.P.; McElmurry, R.T.; Bell, S.; Xia, L.; Zhou, N.; Riddle, M.; Schroeder, T.M.; et al. Sarcoma Derived from Cultured Mesenchymal Stem Cells. Stem Cells 2006, 25, 371–379. [Google Scholar] [CrossRef]
- Djouad, F.; Plence, P.; Bony, C.; Tropel, P.; Apparailly, F.; Sany, J.; Noёl, D.; Jorgensen, C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003, 102, 3837–3844. [Google Scholar] [CrossRef]
- Plock, J.A.; Schnider, J.T.; Schweizer, R.; Gorantla, V.S. Are cultured mesenchymal stromal cells an option for immunomodulation in transplantation? Front. Immunol. 2013, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, S.D.; Auny, F.M.; Hassan, Y.; Yesmin, R.; Ara, I.; Mohiuddin, M.S.; Mamun, M.A. Antenatal depression among women with gestational diabetes mellitus: A pilot study. Reprod. Health 2022, 19, 71. [Google Scholar] [CrossRef]
- Mohib, M.; Rabby, S.F.; Paran, T.Z.; Hasan, M.; Ahmed, I.; Hasan, N.; Sagor, A.T.; Mohiuddin, S. Protective role of green tea on diabetic nephropathy—A review. Cogent Biol. 2016, 2, 1248166. [Google Scholar] [CrossRef]
- Choubey, M.; Ranjan, A.; Bora, P.S.; Krishna, A. Protective role of adiponectin against testicular impairment in high-fat diet/streptozotocin-induced type 2 diabetic mice. Biochimie 2020, 168, 41–52. [Google Scholar] [CrossRef]
- Choubey, M.; Ranjan, A.; Krishna, A. Adiponectin/AdipoRs signaling as a key player in testicular aging and associated metabolic disorders. Vitam. Horm. 2021, 115, 611–634. [Google Scholar]
- Choubey, M. Growth Hormone and Insulin-like Growth Factor-I: Novel Insights into the Male Reproductive Health. Growth Disord. Acromegaly 2020, 6, 113–128. [Google Scholar] [CrossRef] [Green Version]


| Disease | The Type of Stem Cell | Effect/Mechanism of Action | Ref. |
|---|---|---|---|
| Alzheimer’s disease | NSC | Reduced neuroinflammation. Increased neurogenesis, cognitive function, and synaptogenesis, by secretion of neuroprotective agents. Enhance secretion neurogenerative growth factors such as vascular endothelial growth factor (VEGF), nerve growth factor (NGF), Brain derived neurotrophic factor (BDNF), and insulin growth factor-1 (IGF-1). | [21,22,23,24,25,26,27,28,29,30] |
| ESC | Continuous production of cholinergic neurons Cognitive function restoration | [31] | |
| MCSC | Improvement in neuronal differentiation, neurogenesis, synaptogenesis Improvement in locomotor and cognitive functions | [32,33,34] | |
| Amyotrophic Lateral Sclerosis | PSC | Differentiate into motor neurons | [35] |
| ESC | Differentiate into motor neurons | [35] | |
| MSC | Neuroprotective effects Increases muscle strength | [35] | |
| NSC | Reduction of progression of diseases Increases muscle strength Neuroprotective effects | [35] | |
| PSC | Differentiate into motor neurons | [36] | |
| Parkinson disease | HFSC | Re-innervation of the affected areas by dopaminergic actions. | [37] |
| NSC | Reproduction of neurons by dopaminergic actions | [38,39] | |
| Huntington’s disease | NSC | Differentiation of progenitor cells into neural cells | [38] |
| Reduction of degeneration | [40] | ||
| HFSC | Improvement in the behavior | [41] | |
| Stroke | MSC | Improvement of movement | [42] |
| NSC | Neuroprotection | [42,43] | |
| ESC | Recovery from the disease and improved movement Neuroprotection | [44,45] | |
| Spinal cord injury | ESC | Recovery from injury | [46] |
| Multipotent neural precursor formation | [47] | ||
| NSC | Re-myelination | [48] | |
| Promote neuroprotection | [49] | ||
| Recovery from injury | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, S.; Choubey, M.; Mohib, M.M.; Arbee, S.; Sagor, M.A.T.; Mohiuddin, M.S. Stem Cell Therapy in Diabetic Polyneuropathy: Recent Advancements and Future Directions. Brain Sci. 2023, 13, 255. https://doi.org/10.3390/brainsci13020255
Akter S, Choubey M, Mohib MM, Arbee S, Sagor MAT, Mohiuddin MS. Stem Cell Therapy in Diabetic Polyneuropathy: Recent Advancements and Future Directions. Brain Sciences. 2023; 13(2):255. https://doi.org/10.3390/brainsci13020255
Chicago/Turabian StyleAkter, Shamima, Mayank Choubey, Mohammad Mohabbulla Mohib, Shahida Arbee, Md Abu Taher Sagor, and Mohammad Sarif Mohiuddin. 2023. "Stem Cell Therapy in Diabetic Polyneuropathy: Recent Advancements and Future Directions" Brain Sciences 13, no. 2: 255. https://doi.org/10.3390/brainsci13020255
APA StyleAkter, S., Choubey, M., Mohib, M. M., Arbee, S., Sagor, M. A. T., & Mohiuddin, M. S. (2023). Stem Cell Therapy in Diabetic Polyneuropathy: Recent Advancements and Future Directions. Brain Sciences, 13(2), 255. https://doi.org/10.3390/brainsci13020255