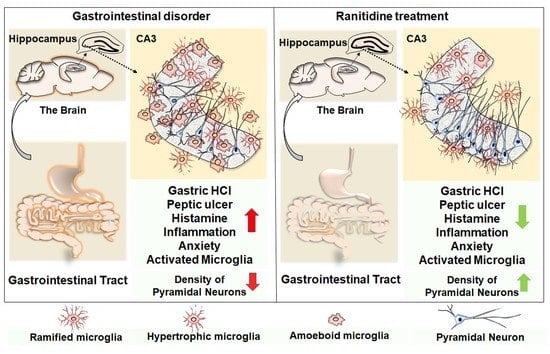
Ranitidine Alleviates Anxiety-like Behaviors and Improves the Density of Pyramidal Neurons upon Deactivation of Microglia in the CA3 Region of the Hippocampus in a Cysteamine HCl-Induced Mouse Model of Gastrointestinal Disorder
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Experimental Animals
2.2. Open Field Test
2.3. Light and Dark Box Test
2.4. Elevated Plus Maze
2.5. Perfusion of Animals and Brain Tissue Processing
2.6. Nissl Staining
2.7. Immunohistochemistry of Microglia
2.8. Statistical Analyses
3. Results
3.1. Ranitidine Treatment Increased the Locomotory Exploration but Minimized Anxiety-Related Behaviours of Mice in the Cysteamine HCl-Treated Group in OFT
3.2. Ranitidine Treatment Reduced Preference-Based and Unconditioned Anxiety-like Behaviors in Cysteamine HCl-Treated Mice in the LDBT
3.3. Ranitidine Treatment Reduced Acrophobia-Related Anxiety-like Behaviors in Cysteamine HCl-Treated Mice in the EPM
3.4. Ranitidine Treatment Increased the Neuronal Density in the CA3 Regions of the Hippocampi in the Brains of Cysteamine HCl-Treated Mice
3.5. Ranitidine Treatment Attenuated Cysteamine HCl-Induced Activation of Microglia in the Hippocampal CA3 Region of the Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bandelow, B.; Michaelis, S. Epidemiology of Anxiety Disorders in the 21st Century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [CrossRef]
- Fikree, A.; Byrne, P. Management of Functional Gastrointestinal Disorders. Clin. Med. (Lond.) 2021, 21, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Shah, E.; Rezaie, A.; Riddle, M.; Pimentel, M. Psychological Disorders in Gastrointestinal Disease: Epiphenomenon, Cause or Consequence? Ann. Gastroenterol. 2014, 27, 224–230. [Google Scholar]
- Spiegel, B.M.R.; Khanna, D.; Bolus, R.; Agarwal, N.; Khanna, P.; Chang, L. Understanding Gastrointestinal Distress: A Framework for Clinical Practice. Am. J. Gastroenterol. 2011, 106, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Sri Rethinavel, H.; Selvaraj, D.B.; Balakrishnan, S.J.; Vergil Andrews, J.F.; Joseph, J.H.M.; Kandasamy, M. Omeprazole Treatment Manifests Anxiolytic Effects in a Cysteamine Hydrochloride Induced Mouse Model of Gastrointestinal Disorder. Heliyon 2022, 8, e09787. [Google Scholar] [CrossRef]
- Hough, L.B. Histamine Actions in the Central Nervous System. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Lippincot-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Jutel, M.; Akdis, M.; Akdis, C.A. Histamine, Histamine Receptors and Their Role in Immune Pathology. Clin. Exp. Allergy 2009, 39, 1786–1800. [Google Scholar] [CrossRef]
- Smolinska, S.; Jutel, M.; Crameri, R.; O’Mahony, L. Histamine and Gut Mucosal Immune Regulation. Allergy 2014, 69, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Borriello, F.; Iannone, R.; Marone, G. Histamine Release from Mast Cells and Basophils. Handb. Exp. Pharmacol. 2017, 241, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the Nervous System. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef] [PubMed]
- Shamburek, R.D.; Schubert, M.L. Control of Gastric Acid Secretion. Histamine H2-Receptor Antagonists and H+K(+)-ATPase Inhibitors. Gastroenterol. Clin. N. Am. 1992, 21, 527–550. [Google Scholar] [CrossRef]
- Anton, A.H.; Woodward, E.R. High Levels of Blood Histamine and Peptic Ulcer. Arch. Surg. 1966, 92, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Passani, M.B.; Panula, P.; Lin, J.-S. Histamine in the Brain. Front. Syst. Neurosci. 2014, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Passani, M.B.; Giannoni, P.; Bucherelli, C.; Baldi, E.; Blandina, P. Histamine in the Brain: Beyond Sleep and Memory. Biochem. Pharmacol. 2007, 73, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Robins, A.H.; Lucke, W.; McFadyen, M.L.; Wright, J.P. Effect of the H2-Receptor Antagonist Ranitidine on Depression and Anxiety in Duodenal Ulcer Patients. Postgrad. Med. J. 1984, 60, 353–355. [Google Scholar] [CrossRef]
- Ozdemir, P.G.; Karadag, A.S.; Selvi, Y.; Boysan, M.; Bilgili, S.G.; Aydin, A.; Onder, S. Assessment of the Effects of Antihistamine Drugs on Mood, Sleep Quality, Sleepiness, and Dream Anxiety. Int. J. Psychiatry Clin. Pract. 2014, 18, 161–168. [Google Scholar] [CrossRef]
- Bachurin, S.; Bukatina, E.; Lermontova, N.; Tkachenko, S.; Afanasiev, A.; Grigoriev, V.; Grigorieva, I.; Ivanov, Y.; Sablin, S.; Zefirov, N. Antihistamine Agent Dimebon as a Novel Neuroprotector and a Cognition Enhancer. Ann. N. Y. Acad. Sci. 2001, 939, 425–435. [Google Scholar] [CrossRef]
- Anand, K.S.; Dhikav, V. Hippocampus in Health and Disease: An Overview. Ann. Indian Acad. Neurol. 2012, 15, 239–246. [Google Scholar] [CrossRef]
- Yesudhas, A.; Roshan, S.A.; Radhakrishnan, R.K.; Abirami, G.P.; Manickam, N.; Selvaraj, K.; Elumalai, G.; Shanmugaapriya, S.; Anusuyadevi, M.; Kandasamy, M. Intramuscular Injection of BOTOX® Boosts Learning and Memory in Adult Mice in Association with Enriched Circulation of Platelets and Enhanced Density of Pyramidal Neurons in the Hippocampus. Neurochem. Res. 2020, 45, 2856–2867. [Google Scholar] [CrossRef]
- Yesudhas, A.; Radhakrishnan, R.K.; Sukesh, A.; Ravichandran, S.; Manickam, N.; Kandasamy, M. BOTOX® Counteracts the Innate Anxiety-Related Behaviours in Correlation with Increased Activities of Key Antioxidant Enzymes in the Hippocampus of Ageing Experimental Mice. Biochem. Biophys. Res. Commun. 2021, 569, 54–60. [Google Scholar] [CrossRef]
- Campbell, S.; MacQueen, G. The Role of the Hippocampus in the Pathophysiology of Major Depression. J. Psychiatry Neurosci. 2004, 29, 417–426. [Google Scholar]
- Lisman, J.; Buzsáki, G.; Eichenbaum, H.; Nadel, L.; Ranganath, C.; Redish, A.D. Viewpoints: How the Hippocampus Contributes to Memory, Navigation and Cognition. Nat. Neurosci. 2017, 20, 1434–1447. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Maren, S. Differential Roles for Hippocampal Areas CA1 and CA3 in the Contextual Encoding and Retrieval of Extinguished Fear. Learn. Mem. 2008, 15, 244–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, C.D. What Is the Functional Significance of Chronic Stress-Induced CA3 Dendritic Retraction Within the Hippocampus? Behav. Cogn. Neurosci. Rev. 2006, 5, 41–60. [Google Scholar] [CrossRef]
- Engin, E.; Smith, K.S.; Gao, Y.; Nagy, D.; Foster, R.A.; Tsvetkov, E.; Keist, R.; Crestani, F.; Fritschy, J.-M.; Bolshakov, V.Y.; et al. Modulation of Anxiety and Fear via Distinct Intrahippocampal Circuits. eLife 2016, 5, e14120. [Google Scholar] [CrossRef] [PubMed]
- Baroody, F.M.; Naclerio, R.M. Antiallergic Effects of H1-Receptor Antagonists. Allergy 2000, 55 (Suppl. 64), 17–27. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.M.; Langtry, H.D.; Brogden, R.N. Ranitidine. An Updated Review of Its Pharmacodynamic and Pharmacokinetic Properties and Therapeutic Use in Peptic Ulcer Disease and Other Allied Diseases. Drugs 1989, 37, 801–870. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Z.; Gao, Y.; Wu, Y.; Li, Z.; Liu, H.; Zhang, C. Bidirectional Crosstalk between Stress-Induced Gastric Ulcer and Depression under Chronic Stress. PLoS ONE 2012, 7, e51148. [Google Scholar] [CrossRef]
- Qian, H.; Shu, C.; Xiao, L.; Wang, G. Histamine and Histamine Receptors: Roles in Major Depressive Disorder. Front. Psychiatry 2022, 13, 825591. [Google Scholar] [CrossRef]
- Patwardhan, M.D.; Muley, M.P.; Manekar, M.S. Anti-Inflammatory Property of Ranitidine, a Specific H2-Receptor Antagonist. Indian J. Physiol. Pharmacol. 1986, 30, 205–209. [Google Scholar]
- Lancaster-Smith, M.J.; Jaderberg, M.E.; Jackson, D.A. Ranitidine in the Treatment of Non-Steroidal Anti-Inflammatory Drug Associated Gastric and Duodenal Ulcers. Gut 1991, 32, 252–255. [Google Scholar] [CrossRef]
- Okajima, K.; Harada, N.; Uchiba, M. Ranitidine Reduces Ischemia/Reperfusion-Induced Liver Injury in Rats by Inhibiting Neutrophil Activation. J. Pharmacol. Exp. Ther. 2002, 301, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Ebrahimzadeh, M.A.; Ahmad-Ashrafi, S.; Karami, M.; Mahdavi, M.R.; Saravi, S.S.S. Hepatoprotective, Antinociceptive and Antioxidant Activities of Cimetidine, Ranitidine and Famotidine as Histamine H2 Receptor Antagonists. Fundam. Clin. Pharmacol. 2011, 25, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.; Vila-Leahey, A.; Pessôa, A.C.; Oldford, S.; Marignani, P.A.; Marshall, J.S. Ranitidine Inhibition of Breast Tumor Growth Is B Cell Dependent and Associated With an Enhanced Antitumor Antibody Response. Front. Immunol. 2018, 9, 1894. [Google Scholar] [CrossRef] [Green Version]
- Werbel, T.; Cohen, P.R. Ranitidine-Associated Sleep Disturbance: Case Report and Review of H2 Antihistamine-Related Central Nervous System Adverse Effects. Cureus 2018, 10, e2414. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.M.; El-Sammad, N.M.; Hassan, S.K.; Madboli, A.E.N.A.; Hashim, A.N.; Moustafa, E.S.; Bakry, S.M.; Elsayed, E.A. Antiulcerogenic Effect of Cuphea Ignea Extract against Ethanol-Induced Gastric Ulcer in Rats. BMC Complementary Altern. Med. 2019, 19, 345. [Google Scholar] [CrossRef]
- Kandasamy, M.; Lehner, B.; Kraus, S.; Sander, P.R.; Marschallinger, J.; Rivera, F.J.; Trümbach, D.; Ueberham, U.; Reitsamer, H.A.; Strauss, O.; et al. TGF-Beta Signalling in the Adult Neurogenic Niche Promotes Stem Cell Quiescence as Well as Generation of New Neurons. J. Cell Mol. Med. 2014, 18, 1444–1459. [Google Scholar] [CrossRef]
- Scaccianoce, S.; Lombardo, K.; Nicolai, R.; Affricano, D.; Angelucci, L. Studies on the Involvement of Histamine in the Hypothalamic-Pituitary-Adrenal Axis Activation Induced by Nerve Growth Factor. Life Sci. 2000, 67, 3143–3152. [Google Scholar] [CrossRef]
- Roberts, F.; Calcutt, C.R. Histamine and the Hypothalamus. Neuroscience 1983, 9, 721–739. [Google Scholar] [CrossRef]
- Matejuk, A.; Vandenbark, A.A.; Offner, H. Cross-Talk of the CNS With Immune Cells and Functions in Health and Disease. Front. Neurol. 2021, 12, 672455. [Google Scholar] [CrossRef]
- Carthy, E.; Ellender, T. Histamine, Neuroinflammation and Neurodevelopment: A Review. Front. Neurosci. 2021, 15, 680214. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, W.; Zeng, X.; Hu, G.; Zhang, H.; He, S.; Zhang, S. Histamine Induces Upregulated Expression of Histamine Receptors and Increases Release of Inflammatory Mediators from Microglia. Mol. Neurobiol. 2014, 49, 1487–1500. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Farzam, K.; Sabir, S.; O’Rourke, M.C. Antihistamines. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Michinaga, S.; Sonoda, K.; Inazuki, N.; Ezaki, M.; Awane, H.; Shimizu, K.; Hishinuma, S.; Mizuguchi, H. Selective Histamine H2 Receptor Agonists Alleviate Blood-Brain Barrier Disruption by Promoting the Expression of Vascular Protective Factors Following Traumatic Brain Injury in Mice. J. Pharmacol. Sci. 2022, 150, 135–145. [Google Scholar] [CrossRef]
- Parsons, M.E. Histamine and the Pathogenesis of Duodenal Ulcer Disease. Gut 1985, 26, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.; Haith, L.R.; Reynolds, E.S. Pathogenesis of Duodenal Ulceration Produced by Cysteamine or Propionitrile. Digest. Dis. Sci. 1979, 24, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Vizuete, M.L.; Merino, M.; Venero, J.L.; Santiago, M.; Cano, J.; Machado, A. Histamine Infusion Induces a Selective Dopaminergic Neuronal Death along with an Inflammatory Reaction in Rat Substantia Nigra. J. Neurochem. 2000, 75, 540–552. [Google Scholar] [CrossRef]
- Konturek, S.J.; Radecki, T.; Brzozowski, T.; Piastucki, I.; Dembińska-Kieć, A.; Zmuda, A. Gastric Cytoprotection by Prostaglandins, Ranitidine, and Probanthine in Rats. Role of Endogenous Prostaglandins. Scand. J. Gastroenterol. 1981, 16, 7–12. [Google Scholar]
- Conchillo, A.; Mola, C.; Navarro, C.; Bravo, L.; Bulbena, O. Cytoprotective and Antisecretory Activity of a Ranitidine-Zinc Complex. Prostaglandins Leukot. Essent. Fatty Acids 1995, 52, 393–397. [Google Scholar] [CrossRef]
- Malagelada, C.; Xifró, X.; Badiola, N.; Sabrià, J.; Rodríguez-Álvarez, J. Histamine H2-Receptor Antagonist Ranitidine Protects Against Neural Death Induced by Oxygen-Glucose Deprivation. Stroke 2004, 35, 2396–2401. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, H.J.; Park, H.-K.; Chung, J.-H. Protective Effect of Histamine H2 Receptor Antagonist Ranitidine against Rotenone-Induced Apoptosis. Neurotoxicology 2009, 30, 1114–1119. [Google Scholar] [CrossRef]
- Threlfell, S.; Exley, R.; Cragg, S.J.; Greenfield, S.A. Constitutive Histamine H2 Receptor Activity Regulates Serotonin Release in the Substantia Nigra. J. Neurochem. 2008, 107, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Russom, M.; Tesfai, D.; Solomon Ghebrenegus, A.; Debesai, M.; Kifle, H.; Bahta, I. Intravenous Ranitidine Injection and Risk of Cardiac Arrest: Medication Errors. Int. J. Risk Saf. Med. 2021, 32, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Lombardi, M.; Gressens, P.; Verderio, C. How to Reprogram Microglia toward Beneficial Functions. Glia 2018, 66, 2531–2549. [Google Scholar] [CrossRef] [PubMed]
- Augusto-Oliveira, M.; Arrifano, G.P.; Lopes-Araújo, A.; Santos-Sacramento, L.; Takeda, P.Y.; Anthony, D.C.; Malva, J.O.; Crespo-Lopez, M.E. What Do Microglia Really Do in Healthy Adult Brain? Cells 2019, 8, 1293. [Google Scholar] [CrossRef] [Green Version]
- Dheen, S.T.; Kaur, C.; Ling, E.-A. Microglial Activation and Its Implications in the Brain Diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.; Beddall, A.C.; Bird, C.C.; Bradfield, J.W.; Brown, I.L.; Burnett, A.K.; Cartwright, R.A.; Davies, J.D.; Edwards, M.S.; Ellis, I.O. Distribution of Leukemia, Lymphoma, and Allied Disease in Parts of Great Britain: Analysis by Administrative Districts and Simulations of Adjacencies. Leukaemia Research Fund 1984 Data Collection Group. Leukemia 1987, 1, 78–81. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvaraj, D.B.; Vergil Andrews, J.F.; Anusuyadevi, M.; Kandasamy, M. Ranitidine Alleviates Anxiety-like Behaviors and Improves the Density of Pyramidal Neurons upon Deactivation of Microglia in the CA3 Region of the Hippocampus in a Cysteamine HCl-Induced Mouse Model of Gastrointestinal Disorder. Brain Sci. 2023, 13, 266. https://doi.org/10.3390/brainsci13020266
Selvaraj DB, Vergil Andrews JF, Anusuyadevi M, Kandasamy M. Ranitidine Alleviates Anxiety-like Behaviors and Improves the Density of Pyramidal Neurons upon Deactivation of Microglia in the CA3 Region of the Hippocampus in a Cysteamine HCl-Induced Mouse Model of Gastrointestinal Disorder. Brain Sciences. 2023; 13(2):266. https://doi.org/10.3390/brainsci13020266
Chicago/Turabian StyleSelvaraj, Divya Bharathi, Jemi Feiona Vergil Andrews, Muthuswamy Anusuyadevi, and Mahesh Kandasamy. 2023. "Ranitidine Alleviates Anxiety-like Behaviors and Improves the Density of Pyramidal Neurons upon Deactivation of Microglia in the CA3 Region of the Hippocampus in a Cysteamine HCl-Induced Mouse Model of Gastrointestinal Disorder" Brain Sciences 13, no. 2: 266. https://doi.org/10.3390/brainsci13020266
APA StyleSelvaraj, D. B., Vergil Andrews, J. F., Anusuyadevi, M., & Kandasamy, M. (2023). Ranitidine Alleviates Anxiety-like Behaviors and Improves the Density of Pyramidal Neurons upon Deactivation of Microglia in the CA3 Region of the Hippocampus in a Cysteamine HCl-Induced Mouse Model of Gastrointestinal Disorder. Brain Sciences, 13(2), 266. https://doi.org/10.3390/brainsci13020266