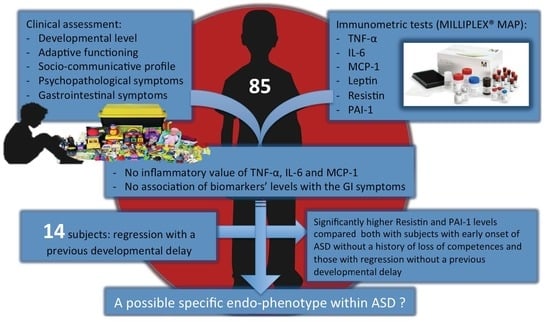
Inflammatory Biomarkers are Correlated with Some Forms of Regressive Autism Spectrum Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Blood Sample Collection
2.3. Cytokine Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Ethics Approval and Consent to Participate
Availability of Data and Material
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGeer, P.L.; Rogers, J.; McGeer, E.G. Inflammation, Antiinflammatory Agents, and Alzheimer’s Disease: The Last 22 Years. J. Alzheimer’s Dis. 2016, 54, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta—Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Datusalia, A.K. Metabolic Stress and Inflammation: Implication in Treatment for Neurological Disorders. CNS Neurol. Disord. Drug Targets 2018, 17, 642–643. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef] [PubMed]
- Fève, B.; Bastard, J.-P. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 305–311. [Google Scholar] [CrossRef]
- Kawazoe, Y.; Naka, T.; Fujimoto, M.; Kohzaki, H.; Morita, Y.; Narazaki, M.; Okumura, K.; Saitoh, H.; Nakagawa, R.; Uchiyama, Y. Signal transducer and activator of transcription (STAT)-induced STAT inhibitor 1 (SSI-1)/suppressor of cytokine signaling 1 (SOCS1) inhibits insulin signal transduction pathway through modulating insulin receptor substrate 1 (IRS-1) phosphorylation. J. Exp. Med. 2001, 193, 263–270. [Google Scholar] [CrossRef]
- Mead, J.; Ashwood, P. Evidence supporting an altered immune response in ASD. Immunol. Lett. 2015, 163, 49–55. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Ataeinia, B.; Keynejad, K.; Abdolalizadeh, A.; Hirbod-Mobarakeh, A.; Rezaei, N. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: Effects of age, gender, and latitude. J. Psychiatr. Res. 2019, 115, 90–102. [Google Scholar] [CrossRef]
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; APA: Washington, DC, USA, 2013. [Google Scholar]
- Bai, D.; Yip, B.H.K.; Windham, G.C.; Sourander, A.; Francis, R.; Yoffe, R.; Glasson, E.; Mahjani, B.; Suominen, A.; Leonard, H.; et al. Association of Genetic and Environmental Factors With Autism in a 5-Country Cohort. JAMA Psychiatry 2019. [Google Scholar] [CrossRef]
- Piven, J.; Elison, J.T.; Zylka, M.J. Toward a conceptual framework for early brain and behavior development in autism. Mol. Psychiatry 2017, 22, 1385–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, R.H.B.; Goldstein, B.I. Inflammation in children and adolescents with neuropsychiatric disorders: A systematic review. J. Am. Acad. Child. Adolesc. Psychiatry 2014, 53, 274–296. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Angelidou, A.; Alysandratos, K.D.; Zhang, B.; Asadi, S.; Francis, K.; Toniato, E.; Kalogeromitros, D. Mast cell activation and autism. Biochim. Biophys. Acta 2012, 1822, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuer, L.S.; Croen, L.A.; Jones, K.L.; Yoshida, C.K.; Hansen, R.L.; Yolken, R.; Zerbo, O.; DeLorenze, G.; Kharrazi, M.; Ashwood, P.; et al. An Exploratory Examination of Neonatal Cytokines and Chemokines as Predictors of Autism Risk: The Early Markers for Autism Study. Biol. Psychiatry 2019, 86, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chadman, K.K.; McCloskey, D.P.; Sheikh, A.M.; Malik, M.; Brown, W.T.; Li, X. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim. Biophys. Acta 2012, 1822, 831–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brainbehav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinmetz, C.C.; Turrigiano, G.G. Tumor necrosis factor-alpha signaling maintains the ability of cortical synapses to express synaptic scaling. J. Neurosci. 2010, 30, 14685–14690. [Google Scholar] [CrossRef]
- Inga Jacome, M.C.; Morales Chacon, L.M.; Vera Cuesta, H.; Maragoto Rizo, C.; Whilby Santiesteban, M.; Ramos Hernandez, L.; Noris Garcia, E.; Gonzalez Fraguela, M.E.; Fernandez Verdecia, C.I.; Vegas Hurtado, Y.; et al. Peripheral Inflammatory Markers Contributing to Comorbidities in Autism. Behav. Sci. 2016, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.N.; Van de Water, J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J. Neuroimmunol. 2011, 232, 196–199. [Google Scholar] [CrossRef] [Green Version]
- Rose, D.R.; Yang, H.; Serena, G.; Sturgeon, C.; Ma, B.; Careaga, M.; Hughes, H.K.; Angkustsiri, K.; Rose, M.; Hertz-Picciotto, I.; et al. Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brainbehav. Immun. 2018, 70, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Vulchanova, L.; Brown, D.R. Stress at the intestinal surface: Catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 2011, 343, 23–32. [Google Scholar] [CrossRef] [PubMed]
- von Kanel, R.; Kudielka, B.M.; Preckel, D.; Hanebuth, D.; Fischer, J.E. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brainbehav. Immun. 2006, 20, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Gorrindo, P.; Williams, K.C.; Lee, E.B.; Walker, L.S.; McGrew, S.G.; Levitt, P. Gastrointestinal dysfunction in autism: Parental report, clinical evaluation, and associated factors. Autism Res. 2012, 5, 101–108. [Google Scholar] [CrossRef]
- Coury, D.L.; Ashwood, P.; Fasano, A.; Fuchs, G.; Geraghty, M.; Kaul, A.; Mawe, G.; Patterson, P.; Jones, N.E. Gastrointestinal conditions in children with autism spectrum disorder: Developing a research agenda. Pediatrics 2012, 130 (Suppl. 2), S160–S168. [Google Scholar] [CrossRef] [Green Version]
- Breece, E.; Paciotti, B.; Nordahl, C.W.; Ozonoff, S.; Van de Water, J.A.; Rogers, S.J.; Amaral, D.; Ashwood, P. Myeloid dendritic cells frequencies are increased in children with autism spectrum disorder and associated with amygdala volume and repetitive behaviors. Brainbehav. Immun. 2013, 31, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, B.J.; Marler, S.; Altstein, L.L.; Lee, E.B.; Mazurek, M.O.; McLaughlin, A.; Macklin, E.A.; McDonnell, E.; Davis, D.J.; Belenchia, A.M.; et al. Associations between cytokines, endocrine stress response, and gastrointestinal symptoms in autism spectrum disorder. Brainbehav. Immun. 2016, 58, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.H.; Rocha, N.P.; Sousa, L.F.; Barbosa, I.G.; Kummer, A.; Teixeira, A.L. Changes in adipokine levels in autism spectrum disorders. Neuropsychobiology 2014, 69, 6–10. [Google Scholar] [CrossRef]
- Pan, W.; Kastin, A.J. Adipokines and the blood-brain barrier. Peptides 2007, 28, 1317–1330. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 71. [Google Scholar] [CrossRef] [Green Version]
- Blardi, P.; de Lalla, A.; D’Ambrogio, T.; Vonella, G.; Ceccatelli, L.; Auteri, A.; Hayek, J. Long-term plasma levels of leptin and adiponectin in Rett syndrome. Clin. Endocrinol. 2009, 70, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Blardi, P.; de Lalla, A.; Ceccatelli, L.; Vanessa, G.; Auteri, A.; Hayek, J. Variations of plasma leptin and adiponectin levels in autistic patients. Neurosci. Lett. 2010, 479, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Kwong, C.; Hansen, R.; Hertz-Picciotto, I.; Croen, L.; Krakowiak, P.; Walker, W.; Pessah, I.N.; Van de Water, J. Brief report: Plasma leptin levels are elevated in autism: Association with early onset phenotype? J. Autism Dev. Disord. 2008, 38, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Valleau, J.C.; Sullivan, E.L. The impact of leptin on perinatal development and psychopathology. J. Chem. Neuroanat. 2014, 61–62, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar]
- Kaser, S.; Kaser, A.; Sandhofer, A.; Ebenbichler, C.F.; Tilg, H.; Patsch, J.R. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem. Biophys. Res. Commun. 2003, 309, 286–290. [Google Scholar] [CrossRef]
- Nehus, E.; Furth, S.; Warady, B.; Mitsnefes, M. Correlates of Resistin in Children with Chronic Kidney Disease: The Chronic Kidney Disease in Children Cohort. J. Pediatr. 2012, 161, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, M.A.; Mousavinejad, E.; Riahi, F.; Mousavinejad, M.; Afsharmanesh, M.R. Increased Serum Levels of Tumor Necrosis Factor-Alpha, Resistin, and Visfatin in the Children with Autism Spectrum Disorders: A Case-Control Study. Neurol. Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Persico, A.M.; Militerni, R.; Bravaccio, C.; Schneider, C.; Melmed, R.; Trillo, S.; Montecchi, F.; Palermo, M.; Pascucci, T.; Puglisi-Allegra, S.; et al. No association between the 4g/5G polymorphism of the plasminogen activator inhibitor-1 gene promoter and autistic disorder. Psychiatr. Genet. 2001, 11, 99–103. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, J.-H.; Kim, J.-H.; Lee, W.-H.; Lee, M.-S.; Suk, K. Plasminogen activator inhibitor type 1 regulates microglial motility and phagocytic activity. J. Neuroinflamm. 2012, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Santocchi, E.; Guiducci, L.; Fulceri, F.; Billeci, L.; Buzzigoli, E.; Apicella, F.; Calderoni, S.; Grossi, E.; Morales, M.A.; Muratori, F. Gut to brain interaction in Autism Spectrum Disorders: A randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry 2016, 16, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S. ADOS-2 Autism Diagnostic Observation Schedule, 2nd ed.; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Griffiths, R. The Griffiths mental developmental scales, revised. Henley: Association for Research in Infant and Child Development; Test Agency: Oxford, UK, 1996. [Google Scholar]
- Sparrow, S.S.; Cicchetti, D.; Balla, D.A. Vineland Adaptive Behavior Scales, 2nd ed.; AGS Publishing: Circle Pines, MN, USA, 2005. [Google Scholar]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA Preschool Forms and Profiles; University of Vermont, Research Center for Children, Youth, Families: Burlington, VT, USA, 2000. [Google Scholar]
- Bodfish, J.W.; Symons, F.J.; Parker, D.E.; Lewis, M.H. Varieties of repetitive behavior in autism: Comparisons to mental retardation. J. Autism Dev. Disord. 2000, 30, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Bailey, A.; Lord, C. The Social Communication Questionnaire: Manual; Western Psychological Services: Los Angeles, CA, USA, 2003. [Google Scholar]
- Schneider, C.K.; Melmed, R.D.; Barstow, L.E.; Enriquez, F.J.; Ranger-Moore, J.; Ostrem, J.A. Oral human immunoglobulin for children with autism and gastrointestinal dysfunction: A prospective, open-label study. J. Autism Dev. Disord. 2006, 36, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.L.; Ozonoff, S.; Krakowiak, P.; Angkustsiri, K.; Jones, C.; Deprey, L.J.; Le, D.N.; Croen, L.A.; Hertz-Picciotto, I. Regression in autism: Prevalence and associated factors in the CHARGE Study. Ambul. Pediatr. 2008, 8, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Geier, D.A.; Geier, M.R. Evaluation of regression in autism spectrum disorder based on parental reports. N. Am. J. Med. Sci. 2014, 6, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monastero, R.N.; Pentyala, S. Cytokines as Biomarkers and Their Respective Clinical Cutoff Levels. Int. J. Inflamm. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Liao, X.; Feng, T.; Wu, Q.; Zhang, J.; Cao, X.; Li, H. Plasma Monocyte Chemoattractant Protein 1 as a Predictive Marker for Sepsis Prognosis: A Prospective Cohort Study. Tohoku J. Exp. Med. 2017, 241, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.A.; Thompson, G.C.; Joffe, A.R.; Blackwood, J.; Martin, D.A.; Brindle, M.; Barkema, H.W.; Jenne, C.N. Cytokines and Chemokines in Pediatric Appendicitis: A Multiplex Analysis of Inflammatory Protein Mediators. Mediat. Inflamm. 2019, 2019. [Google Scholar] [CrossRef]
- Gomez-Fernandez, A.; de la Torre-Aguilar, M.J.; Gil-Campos, M.; Flores-Rojas, K.; Cruz-Rico, M.D.; Martin-Borreguero, P.; Perez-Navero, J.L. Children With Autism Spectrum Disorder With Regression Exhibit a Different Profile in Plasma Cytokines and Adhesion Molecules Compared to Children Without Such Regression. Front. Pediatr. 2018, 6, 264. [Google Scholar] [CrossRef] [Green Version]
- Guloksuz, S.A.; Abali, O.; Aktas Cetin, E.; Bilgic Gazioglu, S.; Deniz, G.; Yildirim, A.; Kawikova, I.; Guloksuz, S.; Leckman, J.F. Elevated plasma concentrations of S100 calcium-binding protein B and tumor necrosis factor alpha in children with autism spectrum disorders. Braz. J. Psychiatry 2017, 39, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Careaga, M.; Rogers, S.; Hansen, R.L.; Amaral, D.G.; Van de Water, J.; Ashwood, P. Immune Endophenotypes in Children With Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Careaga, M.; Van de Water, J.; Ashwood, P. Immune dysfunction in autism: A pathway to treatment. Neurother. J. Am. Soc. Exp. Neurother. 2010, 7, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, V.; Wagner, G.C.; Ming, X. Gastrointestinal dysfunction in children with autism spectrum disorders. Autism Res. 2014, 7, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H.; Geng, L.; Ruby, A.; Reddy, C.; Zimmerman-Bier, B. Evaluation of an association between gastrointestinal symptoms and cytokine production against common dietary proteins in children with autism spectrum disorders. J. Pediatr. 2005, 146, 605–610. [Google Scholar] [CrossRef]
- Ashwood, P.; Anthony, A.; Torrente, F.; Wakefield, A.J. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: Mucosal immune activation and reduced counter regulatory interleukin-10. J. Clin. Immunol. 2004, 24, 664–673. [Google Scholar] [CrossRef]
- Ashwood, P.; Wakefield, A.J. Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J. Neuroimmunol. 2006, 173, 126–134. [Google Scholar] [CrossRef]
- Torrente, F.; Ashwood, P.; Day, R.; Machado, N.; Furlano, R.I.; Anthony, A.; Davies, S.E.; Wakefield, A.J.; Thomson, M.A.; Walker-Smith, J.A.; et al. Small intestinal enteropathy with epithelial IgG and complement deposition in children with regressive autism. Mol. Psychiatry 2002, 7, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Furlano, R.I.; Anthony, A.; Day, R.; Brown, A.; McGarvey, L.; Thomson, M.A.; Davies, S.E.; Berelowitz, M.; Forbes, A.; Wakefield, A.J.; et al. Colonic CD8 and gamma delta T-cell infiltration with epithelial damage in children with autism. J. Pediatr. 2001, 138, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Prosperi, M.; Santocchi, E.; Balboni, G.; Narzisi, A.; Bozza, M.; Fulceri, F.; Apicella, F.; Igliozzi, R.; Cosenza, A.; Tancredi, R.; et al. Behavioral Phenotype of ASD Preschoolers with Gastrointestinal Symptoms or Food Selectivity. J. Autism Dev. Disord. 2017, 47, 3574–3588. [Google Scholar] [CrossRef]
- Masi, A.; Breen, E.J.; Alvares, G.A.; Glozier, N.; Hickie, I.B.; Hunt, A.; Hui, J.; Beilby, J.; Ravine, D.; Wray, J.; et al. Cytokine levels and associations with symptom severity in male and female children with autism spectrum disorder. Mol. Autism 2017, 8, 63. [Google Scholar] [CrossRef]
- Enstrom, A.M.; Onore, C.E.; Van de Water, J.A.; Ashwood, P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brainbehav. Immun. 2010, 24, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Huang, L.; Li, X.; Li, H.; Zhou, Y.; Zhu, H.; Pan, T.; Kendrick, K.M.; Xu, W. Immunological cytokine profiling identifies TNF-alpha as a key molecule dysregulated in autistic children. Oncotarget 2017, 8, 82390–82398. [Google Scholar]
- Gladysz, D.; Krzywdzinska, A.; Hozyasz, K.K. Immune Abnormalities in Autism Spectrum Disorder-Could They Hold Promise for Causative Treatment? Mol. Neurobiol. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napolioni, V.; Ober-Reynolds, B.; Szelinger, S.; Corneveaux, J.J.; Pawlowski, T.; Ober-Reynolds, S.; Kirwan, J.; Persico, A.M.; Melmed, R.D.; Craig, D.W.; et al. Plasma cytokine profiling in sibling pairs discordant for autism spectrum disorder. J. Neuroinflamm. 2013, 10, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordahl, C.W.; Lange, N.; Li, D.D.; Barnett, L.A.; Lee, A.; Buonocore, M.H.; Simon, T.J.; Rogers, S.; Ozonoff, S.; Amaral, D.G. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc. Natl. Acad. Sci. USA 2011, 108, 20195–20200. [Google Scholar] [CrossRef] [Green Version]
- Valvo, G.; Baldini, S.; Retico, A.; Rossi, G.; Tancredi, R.; Ferrari, A.R.; Calderoni, S.; Apicella, F.; Muratori, F.; Santorelli, F.M.; et al. Temporal lobe connects regression and macrocephaly to autism spectrum disorders. Eur. Child. Adolesc. Psychiatry 2016, 25, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Boterberg, S.; Charman, T.; Marschik, P.B.; Bolte, S.; Roeyers, H. Regression in autism spectrum disorder: A critical overview of retrospective findings and recommendations for future research. Neurosci. Biobehav. Rev. 2019, 102, 24–55. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Bedogni, F.; Boterberg, S.; Camfield, C.; Camfield, P.; Charman, T.; Curfs, L.; Einspieler, C.; Esposito, G.; De Filippis, B.; et al. Towards a consensus on developmental regression. Neurosci. Biobehav. Rev. 2019, 107, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Hartel, C.; Adam, N.; Strunk, T.; Temming, P.; Muller-Steinhardt, M.; Schultz, C. Cytokine responses correlate differentially with age in infancy and early childhood. Clin. Exp. Immunol. 2005, 142, 446–453. [Google Scholar] [CrossRef]
- Masi, A.; Quintana, D.S.; Glozier, N.; Lloyd, A.R.; Hickie, I.B.; Guastella, A.J. Cytokine aberrations in autism spectrum disorder: A systematic review and meta-analysis. Mol. Psychiatry 2015, 20, 440–446. [Google Scholar] [CrossRef]
- Mantovani, R.M.; Rocha, N.P.; Magalhaes, D.M.; Barbosa, I.G.; Teixeira, A.L.; Simoes, E.S.A.C. Early changes in adipokines from overweight to obesity in children and adolescents. J. Pediatr. 2016, 92, 624–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Total Sample (n = 85; 100%) | Non-Verbal (n = 46; 54%) | Verbal (n = 39; 46%) | p | p, Age-adjusted | |
|---|---|---|---|---|---|
| AGE (years) mean ± SD | 4.14 ± 1.08 (range 2.18–6.11) | 3.74 ± 0.96 | 4.62 ± 1.02 | <0.0001 | - |
| MALES | 71 (83.5%) | 38 (44.7%) | 33 (38.8%) | NS | - |
| FEMALES | 14 (16.5%) | 8 (9.4%) | 6 (7.1%) | - | |
| Weight (Kg) | 17.70 ± 3.09 | 17.06 ± 3.1 | 18.56 ± 2.89 | 0.026 | NS |
| BMI (Kg/m2) | 15.95 ± 1.66 (range 12.75–21.43) | 16.07 ± 1.74 | 15.82 ± 1.54 | NS | NS |
| Head Circumference (cm) | 51.21 ± 1.69 (range 55–46) | 51.31 ± 1.83 | 51.09 ± 1.54 | NS | NS |
| ADOS-2 CSS Score a (mean ± SD) | |||||
| Social Affect | 6.43 ± 2.05 | 7.06 ± 1.73 | 5.74 ± 2.09 | 0.002 | n.a.* |
| Restricted and Repetitive Behaviors | 8.23 ± 1.46 | 8.56 ± 1.36 | 7.95 ± 1.50 | NS | n.a.* |
| Total | 7.05 ± 1.85 | 7.72 ± 1.50 | 6.41 ± 1.90 | 0.0007 | n.a.* |
| GMDS-ER b (mean ± SD) | |||||
| Performance Quotients | 70.75 ± 23.33 | 61.47 ± 19.42 | 78.75 ± 23.73 | 0.0018 | n.a.* |
| VABS-II c (mean ± SD) Quotients | |||||
| Communication | 50.86 ± 17.79 | 40.76 ± 10.24 | 63.69 ± 17.43 | <0.0001 | n.a.* |
| Daily Living | 66.56 ± 17.07 | 60.46 ± 13.14 | 73.13 ± 18.16 | 0.0002 | n.a.* |
| Socialization | 63.55 ± 15.02 | 57.35 ± 10.36 | 71.15 ± 16.53 | <0.0001 | n.a.* |
| Motor Skills | 71.88 ± 17.85 | 70.89 ± 17.64 | 75.46 ± 16.75 | NS | n.a.* |
| Composite Score | 59.40 ± 19.53 | 52.96 ± 17.52 | 67.23 ± 19.61 | 0.0007 | n.a.* |
| CBCL 1.5-5 d T-score (mean ± SD) | |||||
| Internalizing Problems | 63.85 ± 9.06 | 64.98 ± 8.30 | 62.72 ± 9.64 | NS | NS |
| Externalizing Problems | 57.10 ± 9.09 | 56.71 ± 8.68 | 57.20 ± 9.55 | NS | NS |
| Total Problems | 62.28 ± 10.51 | 62.73 ± 10.68 | 61.69 ± 10.24 | NS | NS |
| Sleep Problems | 58.21 ± 9.11 | 59.62 ± 10.45 | 56.44 ± 6.83 | NS | NS |
| Attention Problems | 64.15 ± 8.21 | 64.66 ± 8.47 | 63.56 ± 7.98 | NS | NS |
| Aggressive Behavior | 56.58 ± 7.13 | 56.27 ± 5.93 | 56.95 ± 8.38 | NS | NS |
| Attention Deficit/Hyperactivity Problems | 59.31 ± 7.70 | 59.58 ± 7.51 | 59.00 ± 8.00 | NS | NS |
| RBS-R e (mean ± SD) | |||||
| Total Score | 19.87 ± 13.87 | 17.67 ± 10.25 | 22.41 ± 16.91 | NS | NS |
| Total Endorsed Score | 12.76 ± 7.27 | 11.91 ± 5.88 | 13.74 ± 8.58 | NS | NS |
| Low Index | 9.44 ± 6.07 | 9.33 ± 5.67 | 9.56 ± 5.59 | NS | NS |
| High Index | 10.25 ± 9.91 | 8.09 ± 7.11 | 12.79 ± 12.04 | 0.028 | 0.028 |
| SCQ f (mean ± SD) | |||||
| Total Score | 14.98 ± 5.90 | 16.72 ± 5.28 | 13.18 ± 6.16 | 0.006 | NS |
| a | b | c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Sample | No-GI 55 Subjects | GI 30 Subjects | p (No-GI vs. GI) | EO ASD | Reg − DD | Reg + DD | ANOVA p Value | NO VERBAL 46 Subjects | VERBAL 39 Subjects | p (No-V vs. V) | |
| N (%) | 85 (100) | 55 (64.7%) | 30 (35.3%) | 57 (67.0) | 14 (16.5) | 14 (16.5) | |||||
| TNF-α, m (SD) pg/mL range 0.74–16.09 | 6.12 (2.40) | 5.84 (2.01) | 6.63 (2.95) | ns | 6.09 (3.16) | 6.76 (3.16) | 5.56 (2.56) | ns | 6.52 (2.57) | 5.63 (2.50) | ns |
| IL-6, m (SD) pg/mL range 0.80–104.00 | 5.99 (16.17) | 4.70 (13.83) | 8.34 (19.80) | ns | 5.74 (14.47) | 10.82 (27.24) | 2.18 (0.90) | ns | 4.67 (6.96) | 7.54 (22.69) | ns |
| CCL2, m (SD) pg/mL range 26.36–451.00 | 127.22 (58.81) | 131.61 (66.86) | 119.16 (39.90) | ns | 126.85 (56.03) | 135.10 (53.15) | 120.84 (76.74) | ns | 125.38 (53.73) | 129.39 (64.95) | ns |
| Leptin, m (SD) pg/mL range 0.03–4.83 | 1.14 (0.89) | 1.19 (0.96) | 1.06 (0.76) | ns | 1.26 (1.01) | 0.96 (0.50) | 0.88 (0.55) | ns | 1.01 (0.80) | 1.30 (0.97) | ns |
| Resistin, m (SD) ng/mL range 8.1–96.8 | 22.89 (13.63) | 24.50 (14.37) | 19.82 (11.74) | 0.032 | 20.97 (10.45) | 18.30 (9.68) | 35.14 (20.75) | 0.0003 (c > a) 0.0007 (c > b) | 23.65 (15.78) | 21.96 (10.60) | ns |
| PAI-1, m (SD) ng/mL range 5.5–91.2 | 26.04 (18.96) | 27.52 (20.27) | 23.24 (16.13) | ns | 23.28 (12.74) | 22.46 (14.04) | 40.67 (33.68) | 0.0018 (c > a) 0.0090 (c > b) | 28.66 (22.86) | 22.88 (12.32) | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosperi, M.; Guiducci, L.; Peroni, D.G.; Narducci, C.; Gaggini, M.; Calderoni, S.; Tancredi, R.; Morales, M.A.; Gastaldelli, A.; Muratori, F.; et al. Inflammatory Biomarkers are Correlated with Some Forms of Regressive Autism Spectrum Disorder. Brain Sci. 2019, 9, 366. https://doi.org/10.3390/brainsci9120366
Prosperi M, Guiducci L, Peroni DG, Narducci C, Gaggini M, Calderoni S, Tancredi R, Morales MA, Gastaldelli A, Muratori F, et al. Inflammatory Biomarkers are Correlated with Some Forms of Regressive Autism Spectrum Disorder. Brain Sciences. 2019; 9(12):366. https://doi.org/10.3390/brainsci9120366
Chicago/Turabian StyleProsperi, Margherita, Letizia Guiducci, Diego G. Peroni, Chiara Narducci, Melania Gaggini, Sara Calderoni, Raffaella Tancredi, Maria Aurora Morales, Amalia Gastaldelli, Filippo Muratori, and et al. 2019. "Inflammatory Biomarkers are Correlated with Some Forms of Regressive Autism Spectrum Disorder" Brain Sciences 9, no. 12: 366. https://doi.org/10.3390/brainsci9120366
APA StyleProsperi, M., Guiducci, L., Peroni, D. G., Narducci, C., Gaggini, M., Calderoni, S., Tancredi, R., Morales, M. A., Gastaldelli, A., Muratori, F., & Santocchi, E. (2019). Inflammatory Biomarkers are Correlated with Some Forms of Regressive Autism Spectrum Disorder. Brain Sciences, 9(12), 366. https://doi.org/10.3390/brainsci9120366