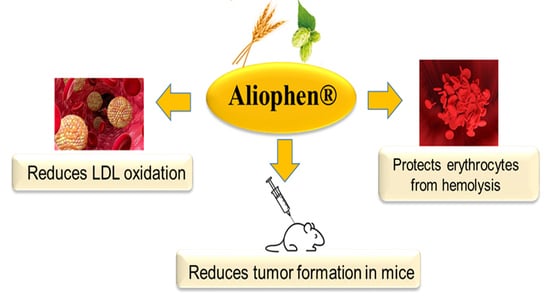
Antioxidant and Chemopreventive Effect of Aliophen® Formulation Based on Malts and Hops
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Aliophen® Preparation
2.3. Analysis of Total Phenolic Content and Antioxidant Capacity
2.4. Extraction of Phenolic Compounds, Identification and Quantification
2.5. Low-Density Lipoprotein Oxidation
2.6. Erythrocytes Isolation and Hemolysis Assay
2.7. Cell Culture and Viability
2.8. Animal Treatment and Evaluations of Pre-Neoplastic Lesions, Polyps and Tumors
2.9. Peroxide Levels in Animal Serum
2.10. Statistics
3. Results
3.1. Polyphenolic Content and In Vitro Antioxidant Capacity of Aliophen®
3.2. Protective Effect of Aliophen® Against LDL Oxidation and Erythrocytes Oxidative Damage
3.2.1. LDL Oxidation
3.2.2. Erythrocytes Oxidative Damage
3.3. Anticancer Effects of Aliophen®
3.3.1. Antiproliferative Effect of Aliophen®
3.3.2. Aliophen® Prevents Preneoplastic Lesions, Polyps, and Tumors
3.3.3. In Vivo Antioxidant Activity of Aliophen®
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Redondo, N.; Nova, E.; Diaz-Prieto, L.E.; Marcos, A. Effects of moderate beer consumption on health. Nutr. Hosp. 2018, 35, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacNaught, N.; Holt, P. Type 1 diabetes and alcohol consumption. Nurs. Stand. 2015, 29, 41–47. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Status Report on Alcohol and Health; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- World Cancer Research FundAmerican Institute for Cancer Research. Continuous Update Project: Alcoholic Drinks and the Risk of Cancer; World Cancer Research FundAmerican Institute for Cancer Research: London, UK, 2018. [Google Scholar]
- Zhang, C.; Zhong, M. Consumption of beer and colorectal cancer incidence: A meta-analysis of observational studies. Cancer Causes Control. 2015, 26, 549–560. [Google Scholar] [CrossRef]
- Ferrari, P.; Licaj, I.; Muller, D.C.; Kragh Andersen, P.; Johansson, M.; Boeing, H.; Weiderpass, E.; Dossus, L.; Dartois, L.; Fagherazzi, G.; et al. Lifetime alcohol use and overall and cause-specific mortality in the European Prospective Investigation into Cancer and nutrition (EPIC) study. BMJ Open 2014, 4, e005245. [Google Scholar] [CrossRef] [Green Version]
- Di Castelnuovo, A.; Costanzo, S.; Bagnardi, V.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Alcohol dosing and total mortality in men and women: An updated meta-analysis of 34 prospective studies. Arch. Intern. Med. 2006, 166, 2437–2445. [Google Scholar] [CrossRef]
- Costanzo, S.; Di Castelnuovo, A.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Cardiovascular and overall mortality risk in relation to alcohol consumption in patients with cardiovascular disease. Circulation 2010, 121, 1951–1959. [Google Scholar] [CrossRef]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martinez, P.; Medina-Remon, A.; Lamuela-Raventos, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [Green Version]
- Chiva-Blanch, G.; Condines, X.; Magraner, E.; Roth, I.; Valderas-Martinez, P.; Arranz, S.; Casas, R.; Martinez-Huelamo, M.; Vallverdu-Queralt, A.; Quifer-Rada, P.; et al. The non-alcoholic fraction of beer increases stromal cell derived factor 1 and the number of circulating endothelial progenitor cells in high cardiovascular risk subjects: A randomized clinical trial. Atherosclerosis 2014, 233, 518–524. [Google Scholar] [CrossRef]
- Osorio-Paz, I.; Brunauer, R.; Alavez, S. Beer and its non-alcoholic compounds in health and disease. Crit. Rev. Food Sci Nutr. 2020, 60, 3492–3505. [Google Scholar] [CrossRef]
- Hamilton, S.R.; Hyland, J.; McAvinchey, D.; Chaudhry, Y.; Hartka, L.; Kim, H.T.; Cichon, P.; Floyd, J.; Turjman, N.; Kessie, G.; et al. Effects of chronic dietary beer and ethanol consumption on experimental colonic carcinogenesis by azoxymethane in rats. Cancer Res. 1987, 47, 1551–1559. [Google Scholar] [PubMed]
- Nozawa, H.; Yoshida, A.; Tajima, O.; Katayama, M.; Sonobe, H.; Wakabayashi, K.; Kondo, K. Intake of beer inhibits azoxymethane-induced colonic carcinogenesis in male Fischer 344 rats. Int. J. Cancer 2004, 108, 404–411. [Google Scholar] [CrossRef] [PubMed]
- de Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaggiari, G.; Cignarelli, A.; Sansone, A.; Baldi, M.; Santi, D. To beer or not to beer: A meta-analysis of the effects of beer consumption on cardiovascular health. PLoS ONE 2020, 15, e0233619. [Google Scholar] [CrossRef]
- Leitao, C.; Marchioni, E.; Bergaentzle, M.; Zhao, M.; Didierjean, L.; Taidi, B.; Ennahar, S. Effects of processing steps on the phenolic content and antioxidant activity of beer. J. Agric. Food Chem. 2011, 59, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Phenol Explorer. Available online: www.phenolexplorer.it (accessed on 25 September 2020).
- Iacomino, G.; Tedesco, I.; Russo, G.L. Biological properties of beer and its components compared to wine. In Beer in Health and Disease Prevention; Preedy, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 1, pp. 483–490. [Google Scholar]
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonca, M.D.C.; Padilha, F.F. Beer Molecules and Its Sensory and Biological Properties: A Review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habschied, K.; Loncaric, A.; Mastanjevic, K. Screening of Polyphenols and Antioxidative Activity in Industrial Beers. Foods 2020, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, D.O.; Curto, A.F.; Guido, L.F. Determination of Phenolic Content in Different Barley Varieties and Corresponding Malts by Liquid Chromatography-diode Array Detection-Electrospray Ionization Tandem Mass Spectrometry. Antioxidants 2015, 4, 563–576. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, M.D.; Moreno, H.; Calvo, J.R. Melatonin present in beer contributes to increase the levels of melatonin and antioxidant capacity of the human serum. Clin. Nutr. 2009, 28, 188–191. [Google Scholar] [CrossRef]
- Alvarez, J.R.M.; Belles, V.V.; Lopez-Jaen, A.B.; Marin, A.V.; Codoner-Franch, P. Effects of alcohol-free beer on lipid profile and parameters of oxidative stress and inflammation in elderly women. Nutrition 2009, 25, 182–187. [Google Scholar] [CrossRef]
- Ambroz, M.; Lnenickova, K.; Matouskova, P.; Skalova, L.; Bousova, I. Antiproliferative Effects of Hop-derived Prenylflavonoids and Their Influence on the Efficacy of Oxaliplatine, 5-fluorouracil and Irinotecan in Human ColorectalC Cells. Nutrients 2019, 11, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerloff, A.; Singer, M.V.; Feick, P. Beer and its non-alcoholic compounds: Role in pancreatic exocrine secretion, alcoholic pancreatitis and pancreatic carcinoma. Int J. Environ. Res. Public Health 2010, 7, 1093–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Armaghany, T.; Wilson, J.D.; Chu, Q.; Mills, G. Genetic alterations in colorectal cancer. Gastrointest. Cancer Res. 2012, 5, 19–27. [Google Scholar] [PubMed]
- Hadjipetrou, A.; Anyfantakis, D.; Galanakis, C.G.; Kastanakis, M.; Kastanakis, S. Colorectal cancer, screening and primary care: A mini literature review. World J. Gastroenterol. 2017, 23, 6049–6058. [Google Scholar] [CrossRef]
- Ranelletti, F.O.; Maggiano, N.; Serra, F.G.; Ricci, R.; Larocca, L.M.; Lanza, P.; Scambia, G.; Fattorossi, A.; Capelli, A.; Piantelli, M. Quercetin inhibits p21-RAS expression in human colon cancer cell lines and in primary colorectal tumors. Int. J. Cancer 2000, 85, 438–445. [Google Scholar] [CrossRef]
- Bouzaiene, N.N.; Jaziri, S.K.; Kovacic, H.; Chekir-Ghedira, L.; Ghedira, K.; Luis, J. The effects of caffeic, coumaric and ferulic acids on proliferation, superoxide production, adhesion and migration of human tumor cells in vitro. Eur. J. Pharmacol. 2015, 766, 99–105. [Google Scholar] [CrossRef]
- Faried, A.; Kurnia, D.; Faried, L.S.; Usman, N.; Miyazaki, T.; Kato, H.; Kuwano, H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int. J. Oncol. 2007, 30, 605–613. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; Liu, H.; Yu, X. Xanthohumol inhibits colorectal cancer cells via downregulation of Hexokinases II-mediated glycolysis. Int. J. Biol. Sci. 2019, 15, 2497–2508. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, K.; Katayama, M.; Sakata, K.; Kuno, T.; Yoshida, K.; Yamada, Y.; Hirose, Y.; Yoshimi, N.; Mori, H. Inhibitory Effects of Chlorogenic Acid on Azoxymethane-induced Colon Carcinogenesis in Male F344 Rats. Asian Pac. J. Cancer Prev. 2002, 3, 163–166. [Google Scholar]
- Wang, X.; Ye, T.; Chen, W.J.; Lv, Y.; Hao, Z.; Chen, J.; Zhao, J.Y.; Wang, H.P.; Cai, Y.K. Structural shift of gut microbiota during chemo-preventive effects of epigallocatechin gallate on colorectal carcinogenesis in mice. World J. Gastroenterol. 2017, 23, 8128–8139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dihal, A.A.; de Boer, V.C.; van der Woude, H.; Tilburgs, C.; Bruijntjes, J.P.; Alink, G.M.; Rietjens, I.M.; Woutersen, R.A.; Stierum, R.H. Quercetin, but not its glycosidated conjugate rutin, inhibits azoxymethane-induced colorectal carcinogenesis in F344 rats. J. Nutr. 2006, 136, 2862–2867. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, H.; Nakao, W.; Zhao, F.; Kondo, K. Dietary supplement of isohumulones inhibits the formation of aberrant crypt foci with a concomitant decrease in prostaglandin E2 level in rat colon. Mol. Nutr. Food Res. 2005, 49, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, H.; Tazumi, K.; Sato, K.; Yoshida, A.; Takata, J.; Arimoto-Kobayashi, S.; Kondo, K. Inhibitory effects of beer on heterocyclic amine-induced mutagenesis and PhIP-induced aberrant crypt foci in rat colon. Mutat. Res. 2004, 559, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef]
- Russo, G.L.; Tedesco, I.; Spagnuolo, C.; Russo, M. Antioxidant polyphenols in cancer treatment: Friend, foe or foil? Semin. Cancer Biol. 2017, 46, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 229, 152–178. [Google Scholar]
- Tedesco, I.; Carbone, V.; Spagnuolo, C.; Minasi, P.; Russo, G.L. Identification and Quantification of Flavonoids from Two Southern Italian Cultivars of Allium cepa L., Tropea (Red Onion) and Montoro (Copper Onion), and Their Capacity to Protect Human Erythrocytes from Oxidative Stress. J. Agric. Food Chem. 2015, 63, 5229–5238. [Google Scholar] [CrossRef]
- Tedesco, I.; Russo, M.; Bilotto, S.; Spagnuolo, C.; Scognamiglio, A.; Palumbo, R.; Nappo, A.; Iacomino, G.; Moio, L.; Russo, G.L. Dealcoholated red wine induces autophagic and apoptotic cell death in an osteosarcoma cell line. Food Chem. Toxicol. 2013, 60, 377–384. [Google Scholar] [CrossRef]
- Wan-Ibrahim, W.I.; Sidik, K.; Kuppusamy, U.R. A high antioxidant level in edible plants is associated with genotoxic properties. Food Chem. 2010, 122, 1139–1144. [Google Scholar] [CrossRef]
- Borrelli, F.; Pagano, E.; Formisano, C.; Piccolella, S.; Fiorentino, A.; Tenore, G.C.; Izzo, A.A.; Rigano, D.; Pacifico, S. Hyssopus officinalis subsp. aristatus: An unexploited wild-growing crop for new disclosed bioactives. Ind. Crops Prod. 2019, 140, 111594. [Google Scholar] [CrossRef]
- Suwalsky, M.; Villena, F.; Gallardo, M.J. In vitro protective effects of resveratrol against oxidative damage in human erythrocytes. Biochim. Biophys. Acta 2015, 1848, 76–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, R.; Collins, S.; Trujillo, J.; McCredie, K.; Ahearn, M.; Tsai, S.; Metzgar, R.; Aulakh, G.; Ting, R.; Ruscetti, F.; et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 1979, 54, 713–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, M.; Moccia, S.; Bilotto, S.; Spagnuolo, C.; Durante, M.; Lenucci, M.S.; Mita, G.; Volpe, M.G.; Aquino, R.P.; Russo, G.L. A Carotenoid Extract from a Southern Italian Cultivar of Pumpkin Triggers Nonprotective Autophagy in Malignant Cells. Oxid. Med. Cell. Longev. 2017, 2017, 7468538. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Aviello, G.; Petrosino, S.; Orlando, P.; Marsicano, G.; Lutz, B.; Borrelli, F.; Capasso, R.; Nigam, S.; Capasso, F.; et al. Increased endocannabinoid levels reduce the development of precancerous lesions in the mouse colon. J. Mol. Med. (Berlin) 2008, 86, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Tedesco, I.; Volpe, M.G.; Bilotto, S.; Russo, M.; Russo, G.L. Cytotoxic Properties of Lyophilized Beers in a Malignant Cell Line. Food Nutr. Sci. 2014, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Tedesco, I.; Nappo, A.; Petitto, F.; Iacomino, G.; Nazzaro, F.; Palumbo, R.; Russo, G.L. Antioxidant and cytotoxic properties of lyophilized beer extracts on HL-60 cell line. Nutr. Cancer 2005, 52, 74–83. [Google Scholar] [CrossRef]
- Martini, D.; Rossi, S.; Biasini, B.; Zavaroni, I.; Bedogni, G.; Musci, M.; Pruneti, C.; Passeri, G.; Ventura, M.; Di Nuzzo, S.; et al. Claimed effects, outcome variables and methods of measurement for health claims proposed under European Community Regulation 1924/2006 in the framework of protection against oxidative damage and cardiovascular health. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 473–503. [Google Scholar] [CrossRef]
- Suzuki, K.; Ito, Y.; Wakai, K.; Kawado, M.; Hashimoto, S.; Toyoshima, H.; Kojima, M.; Tokudome, S.; Hayakawa, N.; Watanabe, Y.; et al. Serum oxidized low-density lipoprotein levels and risk of colorectal cancer: A case-control study nested in the Japan Collaborative Cohort Study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1781–1787. [Google Scholar]
- Bitorina, A.V.; Oligschlaeger, Y.; Shiri-Sverdlov, R.; Theys, J. Low profile high value target: The role of OxLDL in cancer. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158518. [Google Scholar] [CrossRef] [PubMed]
- Zavodnik, L.B.; Zavodnik, I.B.; Lapshyna, E.A.; Buko, V.U.; Bryszewska, M.J. Hypochlorous acidinduced membrane pore formation in red blood cells. Bioelectrochemistry 2002, 58, 157–161. [Google Scholar] [CrossRef]
- Long, L.H.; Clement, M.V.; Halliwell, B. Artifacts in cell culture: Rapid generation of hydrogen peroxide on addition of (-)-epigallocatechin, (-)-epigallocatechin gallate, (+)-catechin, and quercetin to commonly used cell culture media. Biochem. Biophys. Res. Commun. 2000, 273, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Nourooz-Zadeh, J.; Tajaddini-Sarmadi, J.; Wolff, S.P. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal. Biochem. 1994, 220, 403–409. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Freitas, J.; Nogueira, P.; Henriques, S.N.; Carmo, A.M.; Castro, M.A.; Guido, L.F. Xanthohumol inhibits cell proliferation and induces apoptosis in human thyroid cells. Food Chem. Toxicol. 2018, 121, 450–457. [Google Scholar] [CrossRef]
- Chen, W.J.; Lin, J.K. Mechanisms of cancer chemoprevention by hop bitter acids (beer aroma) through induction of apoptosis mediated by Fas and caspase cascades. J. Agric. Food Chem. 2004, 52, 55–64. [Google Scholar] [CrossRef]
- Walden, D.; Kunnimalaiyaan, S.; Sokolowski, K.; Clark, T.G.; Kunnimalaiyaan, M. Antiproliferative and apoptotic effects of xanthohumol in cholangiocarcinoma. Oncotarget 2017, 8, 88069–88078. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.F. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol. Ther. 2009, 8, 1313–1317. [Google Scholar] [CrossRef] [Green Version]
- Global (United States, European Union and China) Functional Foods and Drinks Market. Research Report 2019–2025; MSR2184802; QY Research Group: Selbyville, DE, USA, 2019; p. 121. [Google Scholar]
- QY Research Group. Global Non-Alcoholic Beer Market. Insights, Forecast. to 2025; MSR1800552; QY Research Group: Rowland Heights, CA, USA, 2019; p. 110. [Google Scholar]
- Gerhards, S.; Talaverano, M.I.; Andres, A.I.; Sanchez-Vicente, C.; Lozano, J.; Garcia-Latorre, C.; Petron, M.J.; Rodrigo, S. Different dry hopping and fermentation methods: Influence on beer nutritional quality. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef]
- Quesada-Molina, M.; Munoz-Garach, A.; Tinahones, F.J.; Moreno-Indias, I. A New Perspective on the Health Benefits of Moderate Beer Consumption: Involvement of the Gut Microbiota. Metabolites 2019, 9, 272. [Google Scholar] [CrossRef] [Green Version]
- Aceto, G.M.; Catalano, T.; Curia, M.C. Molecular Aspects of Colorectal Adenomas: The Interplay among Microenvironment, Oxidative Stress, and Predisposition. BioMed Res. Int. 2020, 2020, 1726309. [Google Scholar] [CrossRef] [PubMed]
- David, S.S.; O’Shea, V.L.; Kundu, S. Base-excision repair of oxidative DNA damage. Nature 2007, 447, 941–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol. 2013, 3, 120144. [Google Scholar] [CrossRef] [Green Version]
- Katyama, M.; Yoshimi, N.; Yamada, Y.; Sakata, K.; Kuno, T.; Yoshida, K.; Qiao, Z.; Vihn, P.Q.; Iwasaki, T.; Kobayashi, H.; et al. Preventive effect of fermented brown rice and rice bran against colon carcinogenesis in male F344 rats. Oncol. Rep. 2002, 9, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Navarro, G.; Martinez-Pinilla, E. Hormetic and Mitochondria-Related Mechanisms of Antioxidant Action of Phytochemicals. Antioxidants 2019, 8, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parathodi Illam, S.; Hussain, A.; Elizabeth, A.; Narayanankutty, A.; Raghavamenon, A.C. Natural combination of phenolic glycosides from fruits resists pro-oxidant insults to colon cells and enhances intrinsic antioxidant status in mice. Toxicol. Rep. 2019, 6, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Fortmann, S.P.; Burda, B.U.; Senger, C.A.; Lin, J.S.; Whitlock, E.P. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 159, 824–834. [Google Scholar] [CrossRef]
- Nunez-Sanchez, M.A.; Gonzalez-Sarrias, A.; Romo-Vaquero, M.; Garcia-Villalba, R.; Selma, M.V.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Dietary phenolics against colorectal cancer—From promising preclinical results to poor translation into clinical trials: Pitfalls and future needs. Mol. Nutr. Food Res. 2015, 59, 1274–1291. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Phytochemicals in cancer prevention and therapy: Truth or dare? Toxins 2010, 2, 517–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]







| Samples | Polyphenol Content (µM EqQ) ** | Polyphenol Content (mg EqQ/100 mL) | AA (%) *** |
|---|---|---|---|
| L-Aliophen® * | 2312 ± 580 | 69.87 ± 17.5 | 25.55 ± 7.4 |
| P-Aliophen® * | 1798 ± 450 | 54.34 ± 13.6 | 45.75 ± 12.2 |
| D-Aliophen® * | 1879 ± 470 | 57.1 ± 14.2 | 37.3 ± 10.8 |
| Commercial beers | |||
| alcohol-free | 480 ± 12 | 14.5 ± 0.40 | 4.0 ± 0.03 |
| Regular | 886 ± 49 | 26.8 ± 1.5 | 10.9 ± 0.3 |
| Red | 1188 ± 32 | 35.9 ± 1.0 | 11.8 ± 0.4 |
| Dark | 1356 ± 30 | 41.0 ± 0.9 | 8.5 ± 0.08 |
| Phenolic Compound | Molecular Formula | RT (min) | Molecular Weight | [M − H]− Found (m/z) | [M − H] (m/z) | Relative Amount * (mg/L) |
|---|---|---|---|---|---|---|
| Protocatechuic acid | C7H6O4 | 6.5 | 154.0266 | 153.0197 | 153.0193 | 0.80 ± 0.01 |
| Vanillic acid | C8H8O4 | 7.5 | 168.1467 | 167.0370 | 167.0371 | 1.24 ± 0.02 |
| Catechin | C15H14O6 | 15.7 | 290.0790 | 289.0721 | 289.0717 | 2.09 ± 0.07 |
| Chlorogenic acid | C16H18O9 | 18.2 | 354.0951 | 353.0868 | 353.0878 | 0.88 ± 0.01 |
| p-Hydroxybenzoic acid | C7H6O3 | 19.5 | 138.0317 | 137.0237 | 137.0244 | 0.47 ± 0.03 |
| Caffeic acid | C9H8O4 | 21.0 | 180.0423 | 179.0341 | 179.0350 | 0.49 ± 0.07 |
| Epicatechin | C15H14O6 | 23.7 | 290.0769 | 289.0699 | 289.0696 | 1.45 ± 0.09 |
| p-Coumaric acid | C9H8O3 | 24.4 | 164.0473 | 163.0400 | 163.0393 | 2.53 ± 0.09 |
| m-Coumaric acid | C9H8O3 | 24.9 | 164.0473 | 163.0416 | 163.0400 | 0.51 ± 0.04 |
| Sinapic acid | C11H12O5 | 28.9 | 224.0685 | 223.0604 | 223.0605 | 0.89 ± 0.03 |
| Ferulic acid | C10H10O4 | 29.8 | 194.0579 | 193.0504 | 193.0505 | 4.93 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedesco, I.; Spagnuolo, C.; Bilotto, S.; Izzo, A.A.; Borrelli, F.; Rigano, D.; Russo, M.; Tarricone, F.; Russo, G.L. Antioxidant and Chemopreventive Effect of Aliophen® Formulation Based on Malts and Hops. Antioxidants 2021, 10, 29. https://doi.org/10.3390/antiox10010029
Tedesco I, Spagnuolo C, Bilotto S, Izzo AA, Borrelli F, Rigano D, Russo M, Tarricone F, Russo GL. Antioxidant and Chemopreventive Effect of Aliophen® Formulation Based on Malts and Hops. Antioxidants. 2021; 10(1):29. https://doi.org/10.3390/antiox10010029
Chicago/Turabian StyleTedesco, Idolo, Carmela Spagnuolo, Stefania Bilotto, Angelo A. Izzo, Francesca Borrelli, Daniela Rigano, Maria Russo, Fabrizio Tarricone, and Gian Luigi Russo. 2021. "Antioxidant and Chemopreventive Effect of Aliophen® Formulation Based on Malts and Hops" Antioxidants 10, no. 1: 29. https://doi.org/10.3390/antiox10010029
APA StyleTedesco, I., Spagnuolo, C., Bilotto, S., Izzo, A. A., Borrelli, F., Rigano, D., Russo, M., Tarricone, F., & Russo, G. L. (2021). Antioxidant and Chemopreventive Effect of Aliophen® Formulation Based on Malts and Hops. Antioxidants, 10(1), 29. https://doi.org/10.3390/antiox10010029