Ibogaine-Mediated ROS/Antioxidant Elevation in Isolated Rat Uterus Is β-Adrenergic Receptors and KATP Channels Mediated
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Chemicals
2.3. Isolated Organ Bath Studies
2.4. Measurement of Antioxidant Enzyme Activities
2.5. Data Analysis and Statistical Procedures
3. Results
3.1. Effects of Single Dose of Ibogaine on Uterine Contractions
3.2. Effects of Single Dose of Ibogaine on Uterine Contractions: The Effect of Glibenclamide and Propranolol Pretreatment
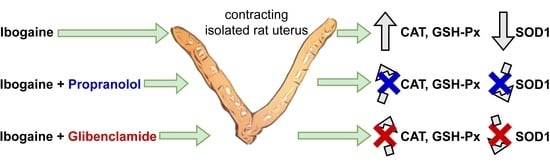
3.3. Effects of a Single Dose of Ibogaine on Antioxidant Enzymes Activity: The Effect of Propranolol and Glibenclamide Pre-Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donnelly, J.R. The Need for Ibogaine in Drug and Alcohol Addiction Treatment. J. Leg. Med. 2011, 32, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Alper, K.R.; Lotsof, H.S.; Frenken, G.M.; Luciano, D.J.; Bastiaans, J. Treatment of acute opioid withdrawal with ibogaine. Am. J. Addict. 1999, 8, 234–242. [Google Scholar] [PubMed]
- Mačiulaitis, R.; Kontrimavičiūtė, V.; Bressolle, F.M.M.; Briedis, V. Ibogaine, an anti-addictive drug: Pharmacology and time to go further in development. A narrative review. Hum. Exp. Toxicol. 2008, 27, 181–194. [Google Scholar] [CrossRef]
- Alper, K.R. Chapter 1 Ibogaine: A review. In The Alkaloids: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 56, pp. 1–38. [Google Scholar]
- Litjens, R.P.W.; Brunt, T.M. How toxic is ibogaine? Clin. Toxicol. 2016, 54, 297–302. [Google Scholar] [CrossRef]
- Vidonja-Uzelac, T.; Tatalovic, N.; Mijovic, M.; Kozelj, G.; Nikolic-Kokic, A.; Dusic, Z.O.; Bresjanac, M.; Blagojević, D. Effects of ibogaine per os application on redox homeostasis in rat liver and erythrocytes. Arch. Biol. Sci. 2019, 71, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Vidonja-Uzelac, T.; Tatalovic, N.; Mijovic, M.; Nikolic-Kokic, A.; Dusic, Z.O.; Bresjanac, M.; Blagojević, D. Effects of ibogaine per os treatment on redox homeostasis in rat kidney. Arch. Biol. Sci. 2019, 71, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Glick, S.D.; Maisonneuve, I.M.; Kitchen, B.A.; Fleck, M.W. Antagonism of alpha 3 beta 4 nicotinic receptors as a strategy to reduce opioid and stimulant self-administration. Eur. J. Pharmacol. 2002, 438, 99–105. [Google Scholar] [CrossRef]
- Leal, M.B.; Michelin, K.; Souza, D.O.; Elisabetsky, E. Ibogaine attenuation of morphine withdrawal in mice: Role of glutamate N-methyl-d-aspartate receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 781–785. [Google Scholar] [CrossRef]
- Paškulin, R.; Jamnik, P.; Živin, M.; Raspor, P.; Štrukelj, B. Ibogaine affects brain energy metabolism. Eur. J. Pharmacol. 2006, 552, 11–14. [Google Scholar] [CrossRef]
- Paškulin, R.; Jamnik, P.; Obermajer, N.; Slavić, M.; Štrukelj, B. Induction of energy metabolism related enzymes in yeast Sac-charomyces cerevisiae exposed to ibogaine is adaptation to acute decrease in ATP energy pool. Eur. J. Pharmacol. 2010, 627, 131–135. [Google Scholar] [CrossRef]
- Paškulin, R.; Jamnik, P.; Danevcic, T.; Kozelj, G.; Krašovec, R.; Krstić-Milosević, D.; Blagojevic, D.; Strukelj, B. Metabolic plasticity and the energy economizing effect of ibogaine, the principal alkaloid of Tabernanthe iboga. J. Ethnopharmacol. 2012, 143, 319–324. [Google Scholar] [CrossRef]
- Nikolić-Kokić, A.; Dusic, Z.O.; Spasojevic, I.; Slavic, M.; Mijuskovic, A.; Paškulin, R.; Miljevic, C.; Spasić, M.B.; Blagojević, D.P. Ex vivo effects of ibogaine on the activity of antioxidative enzymes in human erythrocytes. J. Ethnopharmacol. 2015, 164, 64–70. [Google Scholar] [CrossRef]
- Dusic, Z.O.; Tatalović, N.; Vidonja-Uzelac, T.; Nestorov, J.; Nikolić-Kokić, A.; Mijuskovic, A.; Spasić, M.; Paškulin, R.; Bresjanac, M.; Blagojević, D. The Effects of Ibogaine on Uterine Smooth Muscle Contractions: Relation to the Activity of Antioxidant Enzymes. Oxidative Med. Cell. Longev. 2018, 2018, 5969486. [Google Scholar] [CrossRef] [Green Version]
- Zizzo, M.G.; Mulè, F.; Serio, R. Activation of P2Y receptors by ATP and by its analogue, ADPβS, triggers two calcium signal pathways in the longitudinal muscle of mouse distal colon. Eur. J. Pharmacol. 2008, 595, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hristova, M.; Veith, C.; Habibovic, A.; Lam, Y.-W.; Deng, B.; Geiszt, M.; Janssen-Heininger, Y.M.; van der Vliet, A. Identification of DUOX1-dependent redox signaling through protein S-glutathionylation in airway epithelial cells. Redox Biol. 2014, 2, 436–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiber, A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta 2010, 1797, 897–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, H.; Vervliet, T.; Missiaen, L.; Parys, J.; De Smedt, H.; Bultynck, G. Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim. Biophys. Acta (BBA)-Bioenerg. 2014, 1843, 2164–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekera, P.C.; Wan, T.C.; Gizewski, E.T.; Auchampach, J.A.; Lasley, R.D. Adenosine A1 receptors heterodimerize with β1- and β2-adrenergic receptors creating novel receptor complexes with altered G protein coupling and signaling. Cell. Signal. 2013, 25, 736–742. [Google Scholar] [CrossRef] [Green Version]
- Stallaert, W.; Van Der Westhuizen, E.T.; Schönegge, A.-M.; Plouffe, B.; Hogue, M.; Lukashova, V.; Inoue, A.; Ishida, S.; Aoki, J.; Le Gouill, C.; et al. Purinergic Receptor Transactivation by the β2-Adrenergic Receptor Increases Intracellular Ca2+ in Nonexcitable Cells. Mol. Pharmacol. 2017, 91, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Anikina, T.A.; Zverev, A.A.; Sitdikov, F.G.; Anisimova, I.N. Interaction of adrenergic and purinergic receptors in the regulation of rat myocardial contractility in postnatal ontogeny. Russ. J. Dev. Biol. 2013, 44, 296–301. [Google Scholar] [CrossRef]
- Xu, Q.; Dalic, A.; Fang, L.; Kiriazis, H.; Ritchie, R.; Sim, K.; Gao, X.-M.; Drummond, G.; Sarwar, M.; Zhang, Y.-Y.; et al. Myocardial oxidative stress contributes to transgenic β2-adrenoceptor activation-induced cardiomyopathy and heart failure. Br. J. Pharmacol. 2011, 162, 1012–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lisa, F.; Kaludercic, N.; Paolocci, N. β2-Adrenoceptors, NADPH oxidase, ROS and p38 MAPK: Another ‘radical’ road to heart failure? Br. J. Pharmacol. 2011, 162, 1009–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oreščanin, Z.; Milovanović, S. Effect of L-arginine on the relaxation caused by sodium nitroprusside on isolated rat renal artery. Acta Physiol. Hung. 2006, 93, 271–283. [Google Scholar] [CrossRef]
- Oreščanin-Dušić, Z.; Milovanović, S.; Radojičić, R.; Nikolić-Kokić, A.; Appiah, I.; Slavić, M.; Cutura, N.; Trbojević, S.; Spasić, M.; Blagojević, D. Effects of protamine sulphate on spontaneous and calcium-stimulated contractile activity in the rat uterus are potassium channels-mediated. Gen. Physiol. Biophys. 2009, 28, 143–148. [Google Scholar]
- Appiah, I.; Milovanović, S.; Radojičić, R.; Nikolić-Kokić, A.; Oreščanin-Dušić, Z.; Slavić, M.; Trbojević, S.; Skrbic, R.; Spasic, M.B.; Blagojevic, D. Hydrogen peroxide affects rat uterine contractile activity and endogenous antioxidative defence. Br. J. Pharmacol. 2009, 158, 1932–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appiah, I.; Nikolic-Kokic, A.; Orescanin-Dusic, Z.; Radojicic, R.; Milovanovic, S.; Spasic, M.; Blagojevic, D. Reversible Oxidation of Myometrial Voltage-Gated Potassium Chan-nels with Hydrogen Peroxide. Oxidative Med. Cell. Longev. 2012, 2012, 105820. [Google Scholar] [CrossRef] [Green Version]
- Mijuskovic, A.; Dusic, Z.O.; Nikolić-Kokić, A.; Slavic, M.; Spasić, M.B.; Spasojevic, I.; Blagojevic, D. Comparison of the effects of methanethiol and sodium sulphide on uterine contractile activity. Pharmacol. Rep. 2014, 66, 373–379. [Google Scholar] [CrossRef]
- Mijuskovic, A.; Kokić, A.N.; Dusic, Z.O.; Slavic, M.; Spasić, M.B.; Blagojevic, D. Chloride channels mediate sodium sulphide-induced relaxation in rat uteri. Br. J. Pharmacol. 2015, 172, 3671–3686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearl, S.M.; Hough, L.B.; Boyd, D.L.; Glick, S.D. Sex differences in ibogaine antagonism of morphine-induced locomotor activ-ity and in ibogaine brain levels and metabolism. Pharmacol. Biochem. Behav. 1997, 7, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Kubiliene, A.; Sveikata, A.; Zevzikovas, A.; Sadauskiene, I.; Ivanov, L. Investigation into pharmacokinetic properties of active alkaloid ibogaine and its metabolite noribogaine. Acta Pol. Pharm.-Drug Res. 2017, 74, 1591–1597. [Google Scholar]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Beutler, E. Red Cell Metabolism—A Manual of Biochemical Methods, 3rd ed.; Grune and Stratton: New York, NY, USA, 1982. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 74–77. [Google Scholar]
- Glatzle, D.; Vuilleumier, J.P.; Weber, F.; Decker, K. Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Cell. Mol. Life Sci. 1974, 30, 665–667. [Google Scholar] [CrossRef]
- Hinkle, E.D.; Wiersma, W.; Jurs, G.S. Applied Statistics for Behavioral Sciences, 5th ed.; Houghton Mifflin Company: Boston, MA, USA, 2002. [Google Scholar]
- Zizzo, M.G.; Mule, F.; Serio, R. Evidence that ATP or a related purine is an excitatory neurotransmitter in the longitudinal muscle of mouse distal colon. Br. J. Pharmacol. 2007, 151, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Rizzuto, R.; Pinton, P.; Carrington, W.; Fay, F.S.; Fogarty, K.E.; Lifshitz, L.M.; Tuft, R.A.; Pozzan, T. Close Contacts with the Endoplasmic Reticulum as Determinants of Mitochondrial Ca2+ Responses. Science 1998, 280, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Arnaudeau, S.; Kelley, W.L.; Walsh, J.V.; Demaurex, N. Mitochondria recycle Ca(2+) to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J. Biol. Chem. 2001, 276, 29430–29439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csordás, G.; Renken, C.; Várnai, P.; Walter, L.; Weaver, D.; Buttle, K.F.; Balla, T.; Mannella, C.A.; Hajnóczky, G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006, 174, 915–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzo, P.; Pozzan, T. Mitochondria–endoplasmic reticulum choreography: Structure and signaling dynamics. Trends Cell Biol. 2007, 17, 511–517. [Google Scholar] [CrossRef]
- Ishii, K.; Hirose, K.; Iino, M. Ca 2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep. 2006, 7, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Ward, S.M.; Ordog, T.; Koh, S.D.; Baker, S.A.; Jun, J.Y.; Amberg, G.; Monaghan, K.; Sanders, K.M. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J. Physiol. 2000, 525, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Mundey, M.K.; Blaylock, N.A.; Mason, R.; Glick, S.D.; Maisonneuve, I.M.; Wilson, V.G. Pharmacological comparison of the effect of ibogaine and 18-methoxycoronaridine on isolated smooth muscle from the rat and guinea-pig. Br. J. Pharmacol. 2000, 129, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Strohman, M.J.; Maeda, S.; Hilger, D.; Masureel, M.; Du, Y.; Kobilka, B.K. Local membrane charge regulates β2 adrenergic receptor coupling to Gi3. Nat. Commun. 2019, 10, 2234. [Google Scholar] [CrossRef]
- Alejandro, A.; Eliza, H.S.; Colin, G.N.; Monica, S.-R. Molecular biology of K(ATP) channels and implications for health and disease. IUBMB Life 2009, 61, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, F.M. ATP-sensitive potassium channelopathies: Focus on insulin secretion. J. Clin. Investig. 2005, 115, 2047–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mash, D.C.; Staley, J.K.; Baumann, M.H.; Rothman, R.B.; Hearn, W.L. Identification of a primary metabolite of ibogaine that targets serotonin transporters and elevates serotonin. Life Sci. 1995, 57, PL45–PL50. [Google Scholar] [CrossRef]
- Silverman, D.N.; Nick, H.S. Catalytic pathway of manganese superoxide dismutase by direct observation of superoxide. Methods Enzymol. 2002, 349, 61–74. [Google Scholar]
- Wenzel, P.; Mollnau, H.; Oelze, M.; Schulz, E.; Wickramanayake, J.M.D.; Müller, J.; Schuhmacher, S.; Hortmann, M.; Baldus, S.; Gori, T.; et al. First Evidence for a Crosstalk Between Mitochondrial and NADPH Oxidase-Derived Reactive Oxygen Species in Nitroglycerin-Triggered Vascular Dysfunction. Antioxid. Redox Signal. 2008, 10, 1435–1448. [Google Scholar] [CrossRef]
- Cao, C.; Leng, Y.; Kufe, D. Catalase Activity Is Regulated by c-Abl and Arg in the Oxidative Stress Response. J. Biol. Chem. 2003, 278, 29667–29675. [Google Scholar] [CrossRef] [Green Version]
- Klotz, L.-O.; Sánchez-Ramos, C.; Prieto, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, E.K.; Fridovich, I. The interaction of bovine erythrocyte superoxide dismutase with hydrogenperoxide: Chemi-luminescence and peroxidation. Biochemistry 1975, 14, 5299–5303. [Google Scholar] [CrossRef] [PubMed]
- Mavelli, I.; Ciriolo, M.R.; Rotilio, G. Multiple electrophoretic variants of Cu, Zn superoxide dismutase as expression of the enzyme aging. Effects of H2O2, ascorbate and metal ions. Biochem. Biophys. Res. Commun. 1983, 117, 677–681. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatalović, N.; Vidonja Uzelac, T.; Oreščanin Dušić, Z.; Nikolić-Kokić, A.; Bresjanac, M.; Blagojević, D. Ibogaine-Mediated ROS/Antioxidant Elevation in Isolated Rat Uterus Is β-Adrenergic Receptors and KATP Channels Mediated. Antioxidants 2021, 10, 1792. https://doi.org/10.3390/antiox10111792
Tatalović N, Vidonja Uzelac T, Oreščanin Dušić Z, Nikolić-Kokić A, Bresjanac M, Blagojević D. Ibogaine-Mediated ROS/Antioxidant Elevation in Isolated Rat Uterus Is β-Adrenergic Receptors and KATP Channels Mediated. Antioxidants. 2021; 10(11):1792. https://doi.org/10.3390/antiox10111792
Chicago/Turabian StyleTatalović, Nikola, Teodora Vidonja Uzelac, Zorana Oreščanin Dušić, Aleksandra Nikolić-Kokić, Mara Bresjanac, and Duško Blagojević. 2021. "Ibogaine-Mediated ROS/Antioxidant Elevation in Isolated Rat Uterus Is β-Adrenergic Receptors and KATP Channels Mediated" Antioxidants 10, no. 11: 1792. https://doi.org/10.3390/antiox10111792
APA StyleTatalović, N., Vidonja Uzelac, T., Oreščanin Dušić, Z., Nikolić-Kokić, A., Bresjanac, M., & Blagojević, D. (2021). Ibogaine-Mediated ROS/Antioxidant Elevation in Isolated Rat Uterus Is β-Adrenergic Receptors and KATP Channels Mediated. Antioxidants, 10(11), 1792. https://doi.org/10.3390/antiox10111792