Eccentric Cycling Training Improves Erythrocyte Antioxidant and Oxygen Releasing Capacity Associated with Enhanced Anaerobic Glycolysis and Intracellular Acidosis
Abstract
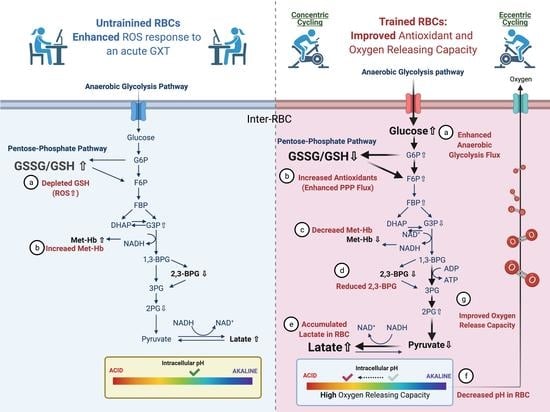
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Protocol and Interventions
2.3. Graded Exercise Tests
2.4. Erythrocyte Isolation and Blood Collection
2.5. Measurement of Reactive Oxygen Species (ROS) Production
2.6. Erythrocyte Intracellular pH
2.7. Oxygen Release Efficacy in Erythrocytes
2.8. Sample Preparation for Targeted Metabolite Identification and Quantification
2.9. Target Metabolite Analysis of Glycolysis Intermediates
2.10. Senescence-Related Biological Markers and Methemoglobin Concentrations in Erythrocytes
2.11. Statistical Analysis
3. Results
3.1. Cardiopulmonary Fitness and Hematological and Blood Gas Parameters
3.2. Pain Scale Scores, Heart Rate and Systolic Blood Pressure during the Training Period
3.3. Erythrocyte Senescence-Related Markers and Antioxidation Capacity
3.4. Target Metabolite Analysis of Glycolysis and Pentose Phosphate Pathway Intermediates
3.5. Erythrocyte O2 Release Capacity in Normal Conditions
3.6. Erythrocyte O2 Release Capacity in Hypoxia Conditions
3.7. Relationships between GSSG/GSH and Lactate/Pyruvate and between Intracellular pH and Lactate Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hody, S.; Croisier, J.L.; Bury, T.; Rogister, B.; Leprince, P. Eccentric Muscle Contractions: Risks and Benefits. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Lastayo, P.C.; Reich, T.E.; Urquhart, M.; Hoppeler, H.; Lindstedt, S.L. Chronic eccentric exercise: Improvements in muscle strength can occur with little demand for oxygen. Am. J. Physiol. 1999, 276, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Haynes, A.; Linden, M.D.; Chasland, L.C.; Nosaka, K.; Maiorana, A.; Dawson, E.A.; Dembo, L.H.; Naylor, L.H.; Green, D.J. Acute impact of conventional and eccentric cycling on platelet and vascular function in patients with chronic heart failure. J. Appl. Physiol. 2017, 122, 1418–1424. [Google Scholar] [CrossRef] [Green Version]
- Gault, M.L.; Willems, M.E. Aging, functional capacity and eccentric exercise training. Aging Dis. 2013, 4, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Nickel, R.; Troncoso, F.; Flores, O.; Gonzalez-Bartholin, R.; Mackay, K.; Diaz, O.; Jalon, M.; Peñailillo, L. Physiological response to eccentric and concentric cycling in patients with chronic obstructive pulmonary disease. Appl. Physiol. Nutr. Metab. 2020, 45, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.; Pearson, S.; Ross, A.; McGuigan, M. Chronic Adaptations to Eccentric Training: A Systematic Review. Sports Med. 2017, 47, 917–941. [Google Scholar] [CrossRef]
- Peñailillo, L.; Mackay, K.; Gonzalez, R.; Valladares, D.; Contreras-Ferrat, A.; Zbinden-Foncea, H.; Nosaka, K. Effects of Eccentric and Concentric Cycling on Markers of Oxidative Stress and Inflammation in Elderly. Med. Sci. Sports Exerc. 2018, 50, 518. [Google Scholar] [CrossRef]
- Peñailillo, L.; Blazevich, A.; Nosaka, K. Energy expenditure and substrate oxidation during and after eccentric cycling. Eur. J. Appl. Physiol. 2014, 114, 805–814. [Google Scholar] [CrossRef]
- Julian, V.; Thivel, D.; Miguet, M.; Pereira, B.; Costes, F.; Coudeyre, E.; Duclos, M.; Richard, R. Eccentric cycling is more efficient in reducing fat mass than concentric cycling in adolescents with obesity. Scand. J. Med. Sci. Sports 2019, 29, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoi, W.; Naito, Y.; Yoshikawa, T. Role of oxidative stress in impaired insulin signaling associated with exercise-induced muscle damage. Free Radic. Biol. Med. 2013, 65, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.V.; de Lima, L.C.R.; Denadai, B.S. Moving forward with backward pedaling: A review on eccentric cycling. Eur. J. Appl. Physiol. 2021, 121, 381–407. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Laaksonen, D.E.; Atalay, M.; Niskanen, L.; Uusitupa, M.; Hanninen, O.; Sen, C.K. Blood glutathione homeostasis as a determinant of resting and exercise-induced oxidative stress in young men. Redox Rep. Commun. Free Radic. Res. 1999, 4, 53–59. [Google Scholar] [CrossRef]
- Marzatico, F.; Pansarasa, O.; Bertorelli, L.; Somenzini, L.; Della Valle, G. Blood free radical antioxidant enzymes and lipid peroxides following long-distance and lactacidemic performances in highly trained aerobic and sprint athletes. J. Sports Med. Phys. Fit. 1997, 37, 235–239. [Google Scholar]
- González-Bartholin, R.; Mackay, K.; Valladares, D.; Zbinden-Foncea, H.; Nosaka, K.; Peñailillo, L. Changes in oxidative stress, inflammation and muscle damage markers following eccentric versus concentric cycling in older adults. Eur. J. Appl. Physiol. 2019, 119, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Elmer, S.J.; McDaniel, J.; Martin, J.C. Alterations in neuromuscular function and perceptual responses following acute eccentric cycling exercise. Eur. J. Appl. Physiol. 2010, 110, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Böning, D.; Hollnagel, C.; Boecker, A.; Göke, S. Bohr shift by lactic acid and the supply of O2 to skeletal muscle. Respir. Physiol. 1991, 85, 231–243. [Google Scholar] [CrossRef]
- Mairbaurl, H. Red blood cells in sports: Effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 2013, 4, 332. [Google Scholar] [CrossRef] [Green Version]
- Chaves, N.A.; Alegria, T.G.P.; Dantas, L.S.; Netto, L.E.S.; Miyamoto, S.; Bonini Domingos, C.R.; da Silva, D.G.H. Impaired antioxidant capacity causes a disruption of metabolic homeostasis in sickle erythrocytes. Free Radic. Biol. Med. 2019, 141, 34–46. [Google Scholar] [CrossRef]
- Rogers, S.C.; Said, A.; Corcuera, D.; McLaughlin, D.; Kell, P.; Doctor, A. Hypoxia limits antioxidant capacity in red blood cells by altering glycolytic pathway dominance. FASEB J. 2009, 23, 3159–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.P.; Keith, W.B. The effects of acute exercise on levels of erythrocyte 2,3-bisphosphoglycerate: A brief review. J. Sports Sci. 1993, 11, 479–484. [Google Scholar] [CrossRef]
- Guesnon, P.; Poyart, C.; Bursaux, E.; Bohn, B. The binding of lactate and chloride ions to human adult hemoglobin. Respir. Physiol. 1979, 38, 115–129. [Google Scholar] [CrossRef]
- Roth, S.; Gmünder, H.; Dröge, W. Regulation of intracellular glutathione levels and lymphocyte functions by lactate. Cell. Immunol. 1991, 136, 95–104. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2017. [Google Scholar]
- Chou, S.L.; Huang, Y.C.; Fu, T.C.; Hsu, C.C.; Wang, J.S. Cycling Exercise Training Alleviates Hypoxia-Impaired Erythrocyte Rheology. Med. Sci. Sports Exerc. 2016, 48, 57–65. [Google Scholar] [CrossRef]
- Tang, H.Y.; Ho, H.Y.; Wu, P.R.; Chen, S.H.; Kuypers, F.A.; Cheng, M.L.; Chiu, D.T.L. Inability to Maintain GSH Pool in G6PD-Deficient Red Cells Causes Futile AMPK Activation and Irreversible Metabolic Disturbance. Antioxid. Redox Sign. 2015, 22, 744–759. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.G.; Smith, D.D.; Stiles, A.R.; Russell, D.W. A comprehensive method for extraction and quantitative analysis of sterols and secosteroids from human plasma. J. Lipid Res. 2012, 53, 1399–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef] [Green Version]
- Sastre, J.; Asensi, M.; Gasco, E.; Pallardo, F.V.; Ferrero, J.A.; Furukawa, T.; Vina, J. Exhaustive physical exercise causes oxidation of glutathione status in blood: Prevention by antioxidant administration. Am. J. Physiol. 1992, 263, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Kobori, H.; Tanigawa, M.; Sato, K.; Yubisui, T.; Hori, H.; Nagata, Y. Methemoglobin reduction by NADH-cytochrome b(5) reductase in Propsilocerus akamusi larvae. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2015, 185, 54–61. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Ahmed, S.B.M.; Elliott, R.L.; Benoit, A.; Alqahtani, S.S.; Ibrahim, M.E.; Bashir, A.H.H.; Alhoufie, S.T.S.; Elhassan, G.O.; Wales, C.C.; et al. The Pentose Phosphate Pathway Dynamics in Cancer and Its Dependency on Intracellular pH. Metabolites 2020, 10, 285. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Muddathir, A.K.; Shayoub, M.E.A. Tumor Acidity as Evolutionary Spite. Cancers 2011, 3, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.Y.; Cheng, M.L.; Chiu, D.T. Glucose-6-phosphate dehydrogenase-from oxidative stress to cellular functions and degenerative diseases. Redox Rep. Commun. Free Radic. Res. 2007, 12, 109–118. [Google Scholar] [CrossRef]
- San-Millán, I.; Stefanoni, D.; Martinez, J.L.; Hansen, K.C.; D’Alessandro, A.; Nemkov, T. Metabolomics of Endurance Capacity in World Tour Professional Cyclists. Front. Physiol. 2020, 11, 578. [Google Scholar] [CrossRef]
- Viskupicova, J.; Blaskovic, D.; Galiniak, S.; Soszyński, M.; Bartosz, G.; Horakova, L.; Sadowska-Bartosz, I. Effect of high glucose concentrations on human erythrocytes in vitro. Redox Biol. 2015, 5, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominelli, P.B.; Wiggins, C.C.; Baker, S.E.; Shepherd, J.R.A.; Roberts, S.K.; Roy, T.K.; Curry, T.B.; Hoyer, J.D.; Oliveira, J.L.; Joyner, M.J. Influence of high affinity haemoglobin on the response to normoxic and hypoxic exercise. J. Physiol. 2020, 598, 1475–1490. [Google Scholar] [CrossRef]
- Richardson, S.L.; Hulikova, A.; Proven, M.; Hipkiss, R.; Akanni, M.; Roy, N.B.A.; Swietach, P. Single-cell O2 exchange imaging shows that cytoplasmic diffusion is a dominant barrier to efficient gas transport in red blood cells. Proc. Natl. Acad. Sci. USA 2020, 117, 10067. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Xiong, Y.; Wang, Y.; Zhao, Y.; Li, Y.; Ren, Y.; Wang, R.; Zhao, M.; Hao, Y.; Liu, H.; et al. Exhaustive-exercise-induced oxidative stress alteration of erythrocyte oxygen release capacity. Canad. J. Physiol. Pharmacol. 2018, 96, 953–962. [Google Scholar] [CrossRef]
- Makhro, A.; Haider, T.; Wang, J.; Bogdanov, N.; Steffen, P.; Wagner, C.; Meyer, T.; Gassmann, M.; Hecksteden, A.; Kaestner, L.; et al. Comparing the impact of an acute exercise bout on plasma amino acid composition, intraerythrocytic Ca2+ handling, and red cell function in athletes and untrained subjects. Cell Calcium 2016, 60, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Calbet, J.; González-Alonso, J.; Helge, J.; Søndergaard, H.; Munch-Andersen, T.; Saltin, B.; Boushel, R. Central and peripheral hemodynamics in exercising humans: Leg vs arm exercise. Scand. J. Med. Sci. Sports 2015, 25, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, 502–516. [Google Scholar] [CrossRef]
- Jensen, F.B. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol. Scand. 2004, 182, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Stringer, W.; Wasserman, K.; Casaburi, R.; Porszasz, J.; Maehara, K.; French, W. Lactic acidosis as a facilitator of oxyhemoglobin dissociation during exercise. J. Appl. Physiol. 1994, 76, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Connes, P.; Caillaud, C.; Mercier, J.; Bouix, D.; Casties, J.F. Injections of recombinant human erythropoietin increases lactate influx into erythrocytes. J. Appl. Physiol. 2004, 97, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomschi, F.; Bizjak, D.A.; Predel, H.-G.; Bloch, W.; Grau, M. Lactate distribution in red blood cells and plasma after a high intensity running exercise in aerobically trained and untrained subjects. J. Hum. Sport Exerc. 2018, 13, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Mairbaurl, H.; Humpeler, E.; Schwaberger, G.; Pessenhofer, H. Training-dependent changes of red cell density and erythrocytic oxygen transport. J. Appl. Physiol. 1983, 55, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Oslund, R.C.; Su, X.; Haugbro, M.; Kee, J.-M.; Esposito, M.; David, Y.; Wang, B.; Ge, E.; Perlman, D.H.; Kang, Y.; et al. Bisphosphoglycerate mutase controls serine pathway flux via 3-phosphoglycerate. Nat. Chem. Biol. 2017, 13, 1081–1087. [Google Scholar] [CrossRef]
- Nishino, T.; Yachie-Kinoshita, A.; Hirayama, A.; Soga, T.; Suematsu, M.; Tomita, M. Dynamic simulation and metabolome analysis of long-term erythrocyte storage in adenine-guanosine solution. PLoS ONE 2013, 8, e71060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böning, D.; Schünemann, H.J.; Maassen, N.; Busse, M.W. Reduction of oxylabile CO2 in human blood by lactate. J. Appl. Physiol. 1993, 74, 710–714. [Google Scholar] [CrossRef]
- Mairbäurl, H.; Schobersberger, W.; Hasibeder, W.; Schwaberger, G.; Gaesser, G.; Tanaka, K.R. Regulation of red cell 2,3-DPG and Hb-O2-affinity during acute exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 55, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Wagner, P.D. Exercise-induced arterial hypoxemia. J. Appl. Physiol. 1999, 87, 1997–2006. [Google Scholar] [CrossRef]
- Rossi, R.; Milzani, A.; Dalle-Donne, I.; Giannerini, F.; Giustarini, D.; Lusini, L.; Colombo, R.; Di Simplicio, P. Different metabolizing ability of thiol reactants in human and rat blood: Biochemical and pharmacological implications. J. Biol. Chem. 2001, 276, 7004–7010. [Google Scholar] [CrossRef] [Green Version]
- Giustarini, D.; Colombo, G.; Garavaglia, M.L.; Astori, E.; Portinaro, N.M.; Reggiani, F.; Badalamenti, S.; Aloisi, A.M.; Santucci, A.; Rossi, R.; et al. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic. Biol. Med. 2017, 112, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Unt, E.; Kairane, C.; Vaher, I.; Zilmer, M. Red blood cell and whole blood glutathione redox status in endurance-trained men following a ski marathon. J. Sports Sci. Med. 2008, 7, 344–349. [Google Scholar] [PubMed]
- Li, X.D.; Sun, G.F.; Zhu, W.B.; Wang, Y.H. Effects of high intensity exhaustive exercise on SOD, MDA, and NO levels in rats with knee osteoarthritis. Genet. Mol. Res. GMR 2015, 14, 12367–12376. [Google Scholar] [CrossRef] [PubMed]
- Erbs, S.; Höllriegel, R.; Linke, A.; Beck Ephraim, B.; Adams, V.; Gielen, S.; Möbius-Winkler, S.; Sandri, M.; Kränkel, N.; Hambrecht, R.; et al. Exercise Training in Patients with Advanced Chronic Heart Failure (NYHA IIIb) Promotes Restoration of Peripheral Vasomotor Function, Induction of Endogenous Regeneration, and Improvement of Left Ventricular Function. Circ. Heart Fail. 2010, 3, 486–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stempak, D.; Dallas, S.; Klein, J.; Bendayan, R.; Koren, G.; Baruchel, S. Glutathione stability in whole blood: Effects of various deproteinizing acids. Ther. Drug Monit. 2001, 23, 542–549. [Google Scholar] [CrossRef]
- Cheng, M.-L.; Lin, J.-F.; Huang, C.-Y.; Li, G.-J.; Shih, L.-M.; Chiu, D.T.-Y.; Ho, H.-Y. Sedoheptulose-1,7-bisphospate Accumulation and Metabolic Anomalies in Hepatoma Cells Exposed to Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 5913635. [Google Scholar] [CrossRef] [Green Version]
- Gutteridge, B.H.J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press Inc.: New York, NY, USA, 2015. [Google Scholar]







| CCT | ECT | CTL | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | ||
| Anthropometrics Characteristics | |||||||
| Age, year | 21.3 ± 0.5 | — | 21.7 ± 0.4 | — | 21.6 ± 0.6 | — | |
| Height, cm | 174 ± 1 | — | 173 ± 2 | — | 175 ± 1 | — | |
| Weight, kg | 67.5 ± 2.3 | 68.4 ± 1.9 | 68.1 ± 1.3 | 67.4 ± 1.5 | 67.2 ± 2.2 | 68.0 ± 2.2 | |
| Hematological Parameters | |||||||
| Red blood cells, 106/µL | 5.10 ± 0.08 | 5.05 ± 0.05 | 5.13 ± 0.06 | 5.06 ± 0.07 | 5.13 ± 0.06 | 5.13 ± 0.07 | |
| Hb, g/dL | 14.9 ± 0.2 | 14.5 ± 0.2 | 15.0 ± 0.4 | 14.9 ± 0.3 | 15.0 ± 0.3 | 14.7 ± 0.3 | |
| Hematocrit, % | 45.4 ± 0.7 | 44.5 ± 0.6 | 46.2 ± 0.6 | 45.3 ± 0.6 | 46.2 ± 0.6 | 45.2 ± 0.5 | |
| i-STAT Parameters | |||||||
| Blood pH, unit | Rest | 7.37 ± 0.02 | 7.37 ± 0.01 | 7.36 ± 0.01 | 7.37 ± 0.01 | 7.36 ± 0.02 | 7.35 ± 0.01 |
| Ex | 7.23 ± 0.02 # | 7.21 ± 0.02 # | 7.19 ± 0.01 # | 7.19 ± 0.01 # | 7.19 ± 0.03 # | 7.19 ± 0.02 # | |
| Blood lactate, mM | Rest | 0.88 ± 0.11 | 0.87 ± 0.11 | 0.87 ± 0.06 | 0.98 ± 0.08 | 0.89 ± 0.09 | 0.93 ± 0.11 |
| Ex | 13.00 ± 0.59 # | 12.66 ± 0.64 # | 13.99 ± 0.51 # | 13.9 ± 0.49 # | 13.16 ± 0.69 # | 12.38 ± 0.73 # | |
| Ventilation Threshold | |||||||
| Work-rate, watt | 125 ± 6 | 151 ± 6 * † | 120 ± 4 | 136 ± 5 * | 121 ± 4.3 | 122 ± 6.8 | |
| VE, L/min | 44.8 ± 2.3 | 52.3 ± 2.7 * | 43.5 ± 1.9 | 51.8 ± 4.3 * | 45.2 ± 1.8 | 46.2 ± 3.9 | |
| VO2, mL/min/kg | 21.3 ± 0.8 | 26.4 ± 1.0 * † | 21.3 ± 0.6 | 23.3 ± 0.5 * | 21.6 ± 1.1 | 21.2 ± 1.2 | |
| % of VO2max, % | 59.8 ± 2.0 | 66.1 ± 2.0 * | 60.6 ± 1.7 | 67.5 ± 1.2 * | 60.92 ± 2.1 | 61.16 ± 1.9 | |
| Maximal Exercise Performance | |||||||
| Work-rate, watt | 191 ± 3 | 223 ± 5 * † | 189 ± 4 | 201 ± 5 * | 188 ± 5 | 190 ± 5 | |
| VE, L/min | 107.4 ± 3.2 | 118.8 ± 2.5 * | 111.9 ± 3.7 | 115.3 ± 2.2 | 109.95 ± 4.7 | 108.3 ± 4.3 | |
| VO2, mL/min/kg | 35.7 ± 1.1 | 40.0 ± 0.8 * | 35.2 ± 0.7 | 34.6 ± 0.7 | 34.1 ± 1.0 | 34.6 ± 1.5 | |
| OUES, unit | 814 ± 23 | 886 ± 20 * | 817 ± 16 | 829 ± 24 | 816 ± 19 | 825 ± 20 | |
| VE-VCO2 slope, unit | 36.8 ± 1.5 | 36.8 ± 1.6 | 37.6 ± 1.9 | 38.5 ± 2.4 | 35.7 ± 1.6 | 35.7 ± 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-C.; Cheng, M.-L.; Tang, H.-Y.; Huang, C.-Y.; Chen, K.-M.; Wang, J.-S. Eccentric Cycling Training Improves Erythrocyte Antioxidant and Oxygen Releasing Capacity Associated with Enhanced Anaerobic Glycolysis and Intracellular Acidosis. Antioxidants 2021, 10, 285. https://doi.org/10.3390/antiox10020285
Huang Y-C, Cheng M-L, Tang H-Y, Huang C-Y, Chen K-M, Wang J-S. Eccentric Cycling Training Improves Erythrocyte Antioxidant and Oxygen Releasing Capacity Associated with Enhanced Anaerobic Glycolysis and Intracellular Acidosis. Antioxidants. 2021; 10(2):285. https://doi.org/10.3390/antiox10020285
Chicago/Turabian StyleHuang, Yu-Chieh, Mei-Ling Cheng, Hsiang-Yu Tang, Chi-Yao Huang, Kuan-Ming Chen, and Jong-Shyan Wang. 2021. "Eccentric Cycling Training Improves Erythrocyte Antioxidant and Oxygen Releasing Capacity Associated with Enhanced Anaerobic Glycolysis and Intracellular Acidosis" Antioxidants 10, no. 2: 285. https://doi.org/10.3390/antiox10020285
APA StyleHuang, Y. -C., Cheng, M. -L., Tang, H. -Y., Huang, C. -Y., Chen, K. -M., & Wang, J. -S. (2021). Eccentric Cycling Training Improves Erythrocyte Antioxidant and Oxygen Releasing Capacity Associated with Enhanced Anaerobic Glycolysis and Intracellular Acidosis. Antioxidants, 10(2), 285. https://doi.org/10.3390/antiox10020285