Polyphenols and IUGR Pregnancies: Effects of the Antioxidant Hydroxytyrosol on Brain Neurochemistry and Development in a Porcine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
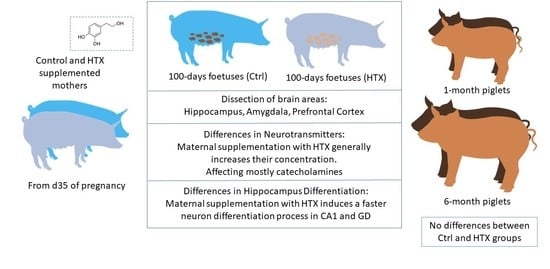
2.2. Animals and Experimental Procedure
2.3. Quantification of Neurotransmitters
2.4. Determination of Parameters Related to Oxidative Stress
2.5. Immunohistochemical Analysis of the Hippocampus
2.6. Image Processing and Analysis
2.7. Statistical Analyses
3. Results
3.1. Effects of Maternal Supplementation with HTX on the Neurotransmitter Profile in Several Brain Areas of the Progeny
3.1.1. Amygdala
3.1.2. Hippocampus
3.1.3. Prefrontal Cortex
3.2. Effects of Maternal Supplementation with HTX on Oxidative Stress Parameters in Fetuses
3.3. Effects of Maternal Supplementation with HTX on Immunohistochemical Markers in the Hippocampus of the Progeny
4. Discussion
4.1. Effects of HTX on the Neurotransmitter Profile in Several Brain Areas
4.2. Effects of HTX on Hippocampal Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2013, 3, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. Biological relevance of extra virgin olive oil polyphenols metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez-Perles, R.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, A. Physiological linkage of gender, bioavailable hydroxytyrosol derivatives, and their metabolites with systemic catecholamine metabolism. Food Funct. 2017, 8, 4570. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana-Perez, C.; Auñon, D.; Garci a-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front. Nutr. 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of olive oil phenols in neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef] [Green Version]
- Peyrol, J.; Riva, C.; Amiot, M.J. Hydroxytyrosol in the prevention of the metabolic syndrome and related disorders. Nutrients 2017, 9, 306. [Google Scholar] [CrossRef]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The fluid aspect of the mediterranean diet in the prevention and management of cardiovascular disease and diabetes: The role of polyphenol content in moderate consumption of wine and olive oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef] [Green Version]
- Zheng, A.; Li, H.; Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Liu, J.; Feng, Z. Maternal hydroxytyrosol administration improves neurogenesis and cognitive function in prenatally stressed offspring. J. Nutr. Biochem. 2015, 26, 190–199. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [Green Version]
- Varghese, N.; Werner, S.; Grimm, A.; Eckert, A. Dietary mitophagy enhancer: A strategy for healthy brain aging? Antioxidants 2020, 9, 932. [Google Scholar] [CrossRef]
- de Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Cerezo, A.B.; de Pablos, R.M.; Krisa, S.; Richard, T.; García-Parrilla, M.C.; Troncoso, A.M. Phenolic compounds characteristic of the mediterranean diet in mitigating microglia-mediated neuroinflammation. Front. Cell. Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef]
- Soni, M.; Prakash, C.; Dabur, R.; Kumar, V. Protective effect of hydroxytyrosol against oxidative stress mediated by arsenic-induced neurotoxicity in rats. Appl. Biochem. Biotechnol. 2018, 186, 27–39. [Google Scholar] [CrossRef]
- Soni, M.; Prakash, C.; Sehwag, S.; Kumar, V. Protective effect of hydroxytyrosol in arsenic-induced mitochondrial dysfunction in rat brain. J. Biochem. Mol. Toxicol. 2017, 31, e21906. [Google Scholar] [CrossRef]
- Casamenti, F.; Stefani, M. Olive polyphenols: New promising agents to combat aging-associated neurodegeneration. Expert Rev. Neurother. 2017, 17, 345–358. [Google Scholar] [CrossRef]
- Aravidou, E.; Eleftheriades, M.; Malamitsi-Puchner, A.; Anagnostopoulos, A.K.; Aravantinos, L.; Dontas, I.; Aravidis, C.; Creatsas, G.; Tsangaris, G.; Chrousos, G.P. Protein expression in the brain of rat offspring in relation to prenatal caloric restriction. J. Matern. Neonatal Med. 2015, 29, 1–8. [Google Scholar] [CrossRef]
- Sawikr, Y.; Yarla, N.S.; Peluso, I.; Kamal, M.A.; Aliev, G.; Bishayee, A. Neuroinflammation in Alzheimer’s disease: The preventive and therapeutic potential of polyphenolic nutraceuticals. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press Inc.: Cambridge, MA, USA, 2017; pp. 33–57. [Google Scholar]
- Perez, M.; Robbins, M.E.; Revhaug, C.; Saugstad, O.D. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free Radic. Biol. Med. 2019, 142, 61–72. [Google Scholar] [CrossRef]
- Rashid, C.S.; Bansal, A.; Simmons, R.A. Oxidative stress, intrauterine growth restriction, and developmental programming of type 2 diabetes. Physiology 2018, 33, 348–359. [Google Scholar] [CrossRef] [Green Version]
- Aljunaidy, M.M.; Morton, J.S.; Cooke, C.L.M.; Davidge, S.T. Prenatal hypoxia and placental oxidative stress: Linkages to developmental origins of cardiovascular disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R395–R399. [Google Scholar] [CrossRef]
- Richter, H.G.; Camm, E.J.; Modi, B.N.; Naeem, F.; Cross, C.M.; Cindrova-Davies, T.; Spasic-Boskovic, O.; Dunster, C.; Mudway, I.S.; Kelly, F.J.; et al. Ascorbate prevents placental oxidative stress and enhances birth weight in hypoxic pregnancy in rats. J. Physiol. 2012, 590, 1377–1387. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, W.; Liu, J. Neurodevelopment in children with intrauterine growth restriction: Adverse effects and interventions. J. Matern. Neonatal Med. 2016, 29, 660–668. [Google Scholar] [CrossRef]
- Yzydorczyk, C.; Armengaud, J.B.; Peyter, A.C.; Chehade, H.; Cachat, F.; Juvet, C.; Siddeek, B.; Simoncini, S.; Sabatier, F.; Dignat-George, F.; et al. Endothelial dysfunction in individuals born after fetal growth restriction: Cardiovascular and renal consequences and preventive approaches. J. Dev. Orig. Health Dis. 2017, 8, 448–464. [Google Scholar] [CrossRef]
- Vuguin, P.M. Animal models for small for gestational age and fetal programing of adult disease. Horm. Res. 2007, 68, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Hovi, P.; Andersson, S.; Eriksson, J.G.; Järvenpää, A.-L.; Strang-Karlsson, S.; Mäkitie, O.; Kajantie, E. Glucose regulation in young adults with very low birth weight. N. Engl. J. Med. 2007, 356, 2053–2063. [Google Scholar] [CrossRef]
- Ross, M.G.; Desai, M. Developmental programming of offspring obesity, adipogenesis, and appetite. Clin. Obstet. Gynecol. 2013, 56, 529–536. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Astiz, S.; Ovilo, C.; Lopez-Bote, C.J.; Torres-Rovira, L.; Barbero, A.; Ayuso, M.; Garcia-Contreras, C.; Vazquez-Gomez, M. Developmental Origins of Health and Disease in swine: Implications for animal production and biomedical research. Theriogenology 2016, 86, 110–119. [Google Scholar] [CrossRef]
- Vázquez-Gómez, M.; Valent, D.; García-Contreras, C.; Arroyo, L.; Óvilo, C.; Isabel, B.; Bassols, A.; González-Bulnes, A. Sex and intrauterine growth restriction modify brain neurotransmitters profile of newborn piglets. Int. J. Dev. Neurosci. 2016, 55, 9–14. [Google Scholar] [CrossRef] [PubMed]
- García-Contreras, C.; Valent, D.; Vázquez-Gómez, M.; Arroyo, L.; Isabel, B.; Astiz, S.; Bassols, A.; Gonzalez-Bulnes, A. Fetal growth-retardation and brain-sparing by malnutrition are associated to changes in neurotransmitters profile. Int. J. Dev. Neurosci. 2017, 57. [Google Scholar] [CrossRef] [PubMed]
- Valent, D.; Yeste, N.; Hernández-Castellano, L.E.; Arroyo, L.; Wu, W.; García-Contreras, C.; Vázquez-Gómez, M.; González-Bulnes, A.; Bendixen, E.; Bassols, A. SWATH-MS quantitative proteomic investigation of intrauterine growth restriction in a porcine model reveals sex differences in hippocampus development. J. Proteom. 2019, 204, 103391. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.A.; Hudspeth, A.J. Principles of Neural Science, 5th ed.; Sydor, A., Lebowitz, H., Eds.; McGraw-Hill Companies, Inc.: New York, NY, USA, 2013. [Google Scholar]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Wixey, J.A.; Lee, K.M.; Miller, S.M.; Goasdoue, K.; Colditz, P.B.; Tracey Bjorkman, S.; Chand, K.K. Neuropathology in intrauterine growth restricted newborn piglets is associated with glial activation and proinflammatory status in the brain 11 Medical and Health Sciences 1109 Neurosciences. J. Neuroinflamm. 2019, 16, 5. [Google Scholar]
- Miller, S.L.; Huppi, P.S.; Mallard, C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016, 594, 807–823. [Google Scholar] [CrossRef] [Green Version]
- Cumberland, A.L.; Palliser, H.K.; Rani, P.; Walker, D.W.; Hirst, J.J. Effects of combined IUGR and prenatal stress on the development of the hippocampus in a fetal guinea pig model. J. Dev. Orig. Health Dis. 2017, 8, 584–596. [Google Scholar] [CrossRef]
- Bauer, R.; Walter, B.; Vorwieger, G.; Bergmann, R.; Füchtner, F.; Brust, P. Intrauterine growth restriction induces up-regulation of cerebral aromatic amino acid decarboxylase activity in newborn piglets: [18F] Fluorodopa positron emission tomographic study. Pediatr. Res. 2001, 49, 474–480. [Google Scholar] [CrossRef] [Green Version]
- Bauer, R.; Walter, B.; Brust, P.; Füchtner, F.; Zwiener, U. Impact of asymmetric intrauterine growth restriction on organ function in newborn piglets. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S40–S49. [Google Scholar] [CrossRef]
- Hernández-Andrade, E.; Cortés-Camberos, A.J.; Díaz, N.F.; Flores-Herrera, H.; García-López, G.; González-Jiménez, M.; Santamaría, A.; Molina-Hernández, A. Altered levels of brain neurotransmitter from new born rabbits with intrauterine restriction. Neurosci. Lett. 2015, 584, 60–65. [Google Scholar] [CrossRef]
- Vucetic, Z.; Totoki, K.; Schoch, H.; Whitaker, K.W.; Hill-Smith, T.; Lucki, I.; Reyes, T.M. Early life protein restriction alters dopamine circuitry. Neuroscience 2010, 168, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Morgane, P.J.; Mokler, D.J.; Galler, J.R. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci. Biobehav. Rev. 2002, 26, 471–483. [Google Scholar] [CrossRef]
- Vazquez-Gomez, M.; Garcia-Contreras, C.; Torres-Rovira, L.; Pesantez, J.L.; Gonzalez-Añover, P.; Gomez-Fidalgo, E.; Sanchez-Sanchez, R.; Ovilo, C.; Isabel, B.; Astiz, S.; et al. Polyphenols and IUGR pregnancies: Maternal hydroxytyrosol supplementation improves prenatal and early-postnatal growth and metabolism of the offspring. PLoS ONE 2017, 12, e0177593. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Gomez, M.; Heras-Molina, A.; Garcia-Contreras, C.; Pesantez-Pacheco, J.L.; Torres-Rovira, L.; Martinez-Fernandez, B.; Gonzalez, J.; Encinas, T.; Astiz, S.; Ovilo, C.; et al. Polyphenols and IUGR pregnancies: Effects of maternal hydroxytyrosol supplementation on postnatal growth, metabolism and body composition of the offspring. Antioxidants 2019, 8, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Barbero, A.; Pesantez, J.L.; Zinellu, A.; Berlinguer, F.; Gonzalez-Añover, P.; Gonzalez, J.; Encinas, T.; Torres-Rovira, L.; et al. Polyphenols and iugr pregnancies: Effects of maternal hydroxytyrosol supplementation on placental gene expression and fetal antioxidant status, DNA-methylation and phenotype. Int. J. Mol. Sci. 2019, 20, 1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Pardo, Z.; Heras-Molina, A.; Pesantez, J.L.; Encinas, T.; Torres-Rovira, L.; Astiz, S.; Nieto, R.; Ovilo, C.; et al. Polyphenols and IUGR pregnancies: Effects of maternal hydroxytyrosol supplementation on hepatic fat accretion and energy and fatty acids profile of fetal tissues. Nutrients 2019, 11, 1534. [Google Scholar] [CrossRef] [Green Version]
- Whittemore, C.T.; Hazzledine, M.J.; Close, W.H. Nutrient Requirement Standards for Pigs; BSAS, British Society of Animal Science: Essex, UK, 2003; ISBN 0906562422. [Google Scholar]
- Gonzalez-Bulnes, A.; Ovilo, C.; Lopez-Bote, C.J.; Astiz, S.; Ayuso, M.; Perez-Solana, M.; Sanchez-Sanchez, R.; Torres-Rovira, L. Gender-specific early postnatal catch-up growth after intrauterine growth retardation by food restriction in swine with obesity/leptin resistance. Reproduction 2012, 144, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, L.; Carreras, R.; Valent, D.; Peña, R.; Mainau, E.; Velarde, A.; Sabrià, J.; Bassols, A. Effect of handling on neurotransmitter profile in pig brain according to fear related behaviour. Physiol. Behav. 2016, 167, 374–381. [Google Scholar] [CrossRef] [Green Version]
- ImageJ. Available online: https://imagej.nih.gov/ij/index.html (accessed on 15 August 2020).
- Heras-Molina, A.; Pesantez-Pacheco, J.L.; Astiz, S.; Garcia-Contreras, C.; Vazquez-Gomez, M.; Encinas, T.; Óvilo, C.; Isabel, B.; Gonzalez-Bulnes, A. Maternal supplementation with polyphenols and omega-3 fatty acids during pregnancy: Effects on growth, metabolism, and body composition of the offspring. Animals 2020, 10, 1946. [Google Scholar] [CrossRef]
- Cabrerizo, S.; De La Cruz, J.P.; López-Villodres, J.A.; Muñoz-Marín, J.; Guerrero, A.; Reyes, J.J.; Labajos, M.T.; González-Correa, J.A. Role of the inhibition of oxidative stress and inflammatory mediators in the neuroprotective effects of hydroxytyrosol in rat brain slices subjected to hypoxia reoxygenation. J. Nutr. Biochem. 2013, 24, 2152–2157. [Google Scholar] [CrossRef]
- Rizzo, M.; Ventrice, D.; Giannetto, F.; Cirinnà, S.; Santagati, N.A.; Procopio, A.; Mollace, V.; Muscoli, C. Antioxidant activity of oleuropein and semisynthetic acetyl-derivatives determined by measuring malondialdehyde in rat brain. J. Pharm. Pharmacol. 2017, 69, 1502–1512. [Google Scholar] [CrossRef]
- Ramírez-Expósito, M.J.; Carrera-González, M.P.; Mayas, M.D.; Martínez-Martos, J.M. Gender differences in the antioxidant response of oral administration of hydroxytyrosol and oleuropein against N-ethyl-N-nitrosourea (ENU)-induced glioma. Food Res. Int. 2021, 140, 110023. [Google Scholar] [CrossRef]
- Zheng, A.; Li, H.; Xu, J.; Cao, K.; Li, H.; Pu, W.; Yang, Z.; Peng, Y.; Long, J.; Liu, J.; et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015, 113, 1667–1676. [Google Scholar] [CrossRef] [Green Version]
- Adebiyi, O.E.; Olopade, J.O.; Olayemi, F.O. Sodium metavanadate induced cognitive decline, behavioral impairments, oxidative stress and down regulation of myelin basic protein in mice hippocampus: Ameliorative roles of β-spinasterol, and stigmasterol. Brain Behav. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, R.; Rasool, A.; Ahmed, M.; Kaleem, U.; Duru, L.N.; Ma, H.; Deng, Y. Synergistic neuroprotective effect of endogenously-produced hydroxytyrosol and synaptic vesicle proteins on pheochromocytoma cell line against salsolinol. Molecules 2020, 25, 1715. [Google Scholar] [CrossRef] [Green Version]
- Benincasa, C.; La Torre, C.; Plastina, P.; Fazio, A.; Perri, E.; Caroleo, M.C.; Gallelli, L.; Cannataro, R.; Cione, E. Hydroxytyrosyl oleate: Improved extraction procedure from olive oil and by-products, and in vitro antioxidant and skin regenerative properties. Antioxidants 2019, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabrià, J.; Tibau, J. The pig as an animal model for human pathologies: A proteomics perspective. Proteom. Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef]
- Burke, W.J.; Li, S.W.; Williams, E.A.; Nonneman, R.; Zahm, D.S. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: Implications for Parkinson’s disease pathogenesis. Brain Res. 2003, 989, 205–213. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Jinsmaa, Y.; Sullivan, P.; Holmes, C.; Kopin, I.J.; Sharabi, Y. 3,4-Dihydroxyphenylethanol (hydroxytyrosol) mitigates the increase in spontaneous oxidation of dopamine during monoamine oxidase inhibition in PC12 cells. Neurochem. Res. 2016, 41, 2173–2178. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Deng, A.; Tang, W.; Ma, J.; Yuan, C.; Ma, J. Hydroxytyrosol induces phase II detoxifying enzyme expression and effectively protects dopaminergic cells against dopamine- and 6-hydroxydopamine induced cytotoxicity. Neurochem. Int. 2016, 96, 113–120. [Google Scholar] [CrossRef]
- Martin, M.A.; Ramos, S.; Granado-Serrano, A.B.; Rodriguez-Ramiro, I.; Trujillo, M.; Bravo, L.; Goya, L. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/ protein kinase B pathways in HepG2 cells. Mol. Nutr. Food Res. 2010, 54, 956–966. [Google Scholar] [CrossRef]
- Gallardo, E.; Madrona, A.; Palma-Valdés, R.; Trujillo, M.; Espartero, J.L.; Santiago, M. The effect of hydroxytyrosol and its nitroderivatives on catechol-O-methyl transferase activity in rat striatal tissue. RSC Adv. 2014, 4, 61086–61091. [Google Scholar] [CrossRef]
- Gallardo, E.; Madrona, A.; Palma-Valdés, R.; Espartero, J.L.; Santiago, M. Effect of intracerebral hydroxytyrosol and its nitroderivatives on striatal dopamine metabolism: A study by in vivo microdialysis. Life Sci. 2015, 134, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Huotari, M.; Passlin, M.; Nordberg, H.L.; Forsberg, M.; Kotisaari, S.; Tuomisto, L.; Shintani, F.; Tanaka, K.F.; Reenilä, I.; Laitinen, K.; et al. Effect of intracerebral 6-nitronoradrenaline, an endogenous catechol-O-methyltransferase (COMT) inhibitor, on striatal dopamine metabolism in anaesthetised rats. J. Neurosci. Methods 2001, 109, 47–52. [Google Scholar] [CrossRef]
- Gallardo, E.; Palma-Valdés, R.; Espartero, J.L.; Santiago, M. In vivo striatal measurement of hydroxytyrosol, and its metabolite (homovanillic alcohol), compared with its derivative nitrohydroxytyrosol. Neurosci. Lett. 2014, 579, 173–176. [Google Scholar] [CrossRef]
- De La Torre, R.; Corella, D.; Castañer, O.; Martínez-González, M.A.; Salas-Salvador, J.; Vila, J.; Estruch, R.; Sorli, J.V.; Arós, F.; Fiol, M.; et al. Protective effect of homovanillyl alcohol on cardiovascular disease and total mortality: Virgin olive oil, wine, and catechol-methylathion. Am. J. Clin. Nutr. 2017, 105, 1297–1304. [Google Scholar] [CrossRef]
- Luo, G.; Huang, Y.; Mo, D.; Ma, N.; Gao, F.; Song, L.; Sun, X.; Xu, X.; Liu, L.; Huo, X.; et al. Tyrosol attenuates pro-inflammatory cytokines from cultured astrocytes and NF-κB activation in in vitro oxygen glucose deprivation. Neurochem. Int. 2018, 121, 140–145. [Google Scholar] [CrossRef]
- Gallardo-Fernández, M.; Hornedo-Ortega, R.; Alonso-Bellido, I.M.; Rodríguez-Gómez, J.A.; Troncoso, A.M.; García-Parrilla, M.C.; Venero, J.L.; Espinosa-Oliva, A.M.; de Pablos, R.M. Hydroxytyrosol decreases LPS- and α-synuclein-induced microglial activation in vitro. Antioxidants 2019, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Deiana, M.; Aruoma, O.I.; Bianchi, M.D.L.P.; Spencer, J.P.E.; Kaur, H.; Halliwell, B.; Aeschbach, R.; Banni, S.; Dessi, M.A.; Corongiu, F.P. Inhibition of peroxynitrite dependent DNA base modification and tyrosine nitration by the extra virgin olive oil-derived antioxidant hydroxytyrosol. Free Radic. Biol. Med. 1999. [Google Scholar] [CrossRef]
- Kesner, R.P.; Lee, I.; Gilbert, P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev. Neurosci. 2004, 15, 333–351. [Google Scholar] [CrossRef]
- Frotscher, M.; Seress, L. Morphological development of the hippocampus. In The Hippocampus Book; Amaral, D.G., Andersen, P., Bliss, T., Morris, R.G.M., O’Keefe, J., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 115–131. [Google Scholar]




| Fetuses | 1 Month | 6 Months | p-Values | ||||
|---|---|---|---|---|---|---|---|
| Treatment | Age | Interaction | |||||
| NA | Ctrl | 44.00 ± 2.42 Aa | 107.93 ± 15.13 Ab | 106.33 ± 5.65 Ab | 0.143 | <0.001 | <0.001 |
| HTX | 31.46 ± 1.84 Ba | 126.21 ± 9.21 Ab | 160.86 ± 18.45 Bb | ||||
| DA | Ctrl | 204.36 ± 12.56 Aa | 496.22 ± 56.40 Ab | 294.06 ± 36.78 Ac | 0.062 | <0.001 | 0.032 |
| HTX | 309.88 ± 32.97 Ba | 404.25 ± 26.16 Ab | 406.63 ± 56.90 Bab | ||||
| DOPAC | Ctrl | 94.84 ± 4.28 Aa | 133.03 ± 15.14 Ab | 114.31 ± 6.48 Aab | <0.001 | 0.008 | 0.072 |
| HTX | 143.09 ± 7.00 Ba | 162.14 ± 13.17 Aa | 139.50 ± 15.03 Aa | ||||
| HVA | Ctrl | 294.93 ± 13.26 Aa | 301.14 ± 43.09 Aa | 270.65 ± 18.18 Aa | 0.002 | <0.001 | 0.001 |
| HTX | 432.63 ± 14.85 Ba | 270.08 ± 19.39 Ab | 345.39 ± 40.52 Ab | ||||
| DOPAC/DA | Ctrl | 0.51 ± 0.02 Aa | 0.28 ± 0.02 Ab | 0.46 ± 0.03 Aa | 0.082 | <0.001 | 0.008 |
| HTX | 0.70 ± 0.17 Aa | 0.41 ± 0.02 Bb | 0.39 ± 0.03 Ab | ||||
| HVA/DA | Ctrl | 1.62 ± 0.09 | 0.63 ± 0.04 | 1.08 ± 0.07 | 0.763 | <0.001 | 0.429 |
| HTX | 2.23 ± 0.43 | 0.70 ± 0.04 | 0.98 ± 0.08 | ||||
| (DOPAC + HVA)/DA | Ctrl | 2.14 ± 0.11 | 0.91 ± 0.06 | 1.54 ± 0.09 | 0.422 | <0.001 | 0.206 |
| HTX | 2.93 ± 0.59 | 1.11 ± 0.06 | 1.37 ± 0.11 | ||||
| DOP total | Ctrl | 594.13 ± 26.24 Aa | 930.39 ± 106.55 Ab | 679.01 ± 59.47 Aa | 0.001 | 0.034 | 0.002 |
| HTX | 885.61 ± 46.65 Ba | 836.47 ± 50.81 Aa | 891.51 ± 103.69 Ba | ||||
| 5-HT | Ctrl | 312.30 ± 14.32 | 731.64 ± 43.08 | 894.76 ± 41.52 | <0.001 | <0.001 | 0.111 |
| HTX | 417.39 ± 17.59 | 886.94 ± 46.18 | 998.98 ± 95.43 | ||||
| 5-HIAA | Ctrl | 113.45 ± 5.28 Aa | 169.36 ± 8.33 Ab | 184.00 ± 7.19 Ab | 0.008 | <0.001 | <0.001 |
| HTX | 163.29 ± 5.73 Ba | 196.31 ± 12.09 Aa | 183.75 ± 18.56 Aa | ||||
| 5-HIAA/5-HT | Ctrl | 0.39 ± 0.02 | 0.24 ± 0.01 | 0.21 ± 0.01 | 0.321 | <0.001 | 0.059 |
| HTX | 0.41 ± 0.02 | 0.23 ± 0.02 | 0.18 ± 0.01 | ||||
| IND total | Ctrl | 425.75 ± 17.86 Aa | 901.00 ± 48.81 Ab | 1078.76 ± 45.48 Ab | <0.001 | <0.001 | 0.021 |
| HTX | 580.68 ± 21.36 Ba | 1083.25 ± 51.18 Bb | 1182.73 ± 111.92 Ab | ||||
| Fetuses | 1 Month | 6 Months | p-Values | ||||
|---|---|---|---|---|---|---|---|
| Treatment | Age | Interaction | |||||
| NA | Ctrl | 60.71 ± 3.28 Aa | 91.21 ± 3.88 Ab | 110.24 ± 3.98 Ab | 0.096 | <0.001 | 0.001 |
| HTX | 81.25 ± 3.89 Ba | 92.42 ± 4.64 Aab | 106.90 ± 7.34 Ab | ||||
| DA | Ctrl | 97.87 ± 9.25 | 18.55 ± 2.31 | 48.99 ± 3.78 | 0.643 | <0.001 | 0.312 |
| HTX | 88.40 ± 4.64 | 19.56 ± 1.88 | 42.76 ± 3.96 | ||||
| DOPAC | Ctrl | 54.37 ± 6.60 Aa | 16.51 ± 2.66 Ab | 28.57 ± 0.67 Aa | 0.002 | <0.001 | 0.023 |
| HTX | 79.54 ± 8.64 Ba | 19.95 ± 2.32 Ab | 31.61 ± 1.60 Ac | ||||
| HVA | Ctrl | 209.07 ± 13.43 Aa | 35.91 ± 1.97 Ab | 40.94 ± 1.11 Ab | 0.024 | <0.001 | 0.015 |
| HTX | 275.01 ± 13.60 Ba | 36.12 ± 2.33 Ab | 46.74 ± 3.01 Ac | ||||
| DOPAC/DA | Ctrl | 0.79 ± 0.10 | 2.04 ± 0.91 | 0.66 ± 0.04 | 0.037 | 0.001 | 0.066 |
| HTX | 1.04 ± 0.15 | 1.10 ± 0.10 | 0.80 ± 0.06 | ||||
| HVA/DA | Ctrl | 2.60 ± 0.18 Aa | 4.50 ± 1.77 Aa | 0.95 ± 0.06 Ab | 0.208 | <0.001 | 0.004 |
| HTX | 3.22 ± 0.13 Ba | 2.08 ± 0.13 Ab | 1.20 ± 0.10 Ac | ||||
| (DOPAC + HVA)/DA | Ctrl | 3.40 ± 0.26 Aa | 6.54 ± 2.68 Aa | 1.61 ± 0.10 Ab | 0.100 | <0.001 | 0.011 |
| HTX | 4.26 ± 0.22 Ba | 3.18 ± 0.18 Ab | 2.00 ± 0.15 Ac | ||||
| DOP total | Ctrl | 361.31 ± 22.42 | 70.97 ± 4.77 | 118.50 ± 4.00 | 0.063 | <0.001 | 0.061 |
| HTX | 442.95 ± 20.04 | 75.64 ± 5.34 | 121.11 ± 6.22 | ||||
| 5-HT | Ctrl | 177.71 ± 7.96 | 264.34 ± 16.57 | 231.35 ± 11.55 | 0.203 | <0.001 | 0.216 |
| HTX | 213.97 ± 10.95 | 274.56 ± 16.94 | 235.98 ± 21.42 | ||||
| 5-HIAA | Ctrl | 88.67 ± 4.15 | 94.39 ± 5.23 | 93.66 ± 3.61 | 0.414 | 0.475 | 0.547 |
| HTX | 94.24 ± 3.06 | 94.21 ± 5.11 | 96.70 ± 4.84 | ||||
| 5-HIAA/5-HT | Ctrl | 0.52 ± 0.02 | 0.37 ± 0.02 | 0.42 ± 0.02 | 0.502 | <0.001 | 0.410 |
| HTX | 0.47 ± 0.02 | 0.36 ± 0.02 | 0.45 ± 0.03 | ||||
| IND total | Ctrl | 266.38 ± 11.14 | 358.73 ± 20.38 | 325.01 ± 13.17 | 0.209 | <0.001 | 0.278 |
| HTX | 308.21 ± 13.05 | 368.77 ± 19.72 | 332.68 ± 23.46 | ||||
| Fetuses | 1 Month | 6 Months | p-Values | ||||
|---|---|---|---|---|---|---|---|
| Treatment | Age | Interaction | |||||
| NA | Ctrl | 26.97 ± 1.19 Aa | 85.57 ± 4.40 Ab | 99.41 ± 4.68 Ab | 0.067 | <0.001 | 0.003 |
| HTX | 19.92 ± 0.98 Ba | 88.78 ± 4.49 Ab | 101.17 ± 6.01 Ac | ||||
| DA | Ctrl | 91.35 ± 11.56 Aa | 18.55 ± 5.72 Ab | 9.22 ± 1.26 Ab | 0.032 | <0.001 | <0.001 |
| HTX | 19.93 ± 0.56 Ba | 14.08 ± 1.53 Aab | 10.33 ± 1.63 Ab | ||||
| DOPAC | Ctrl | 17.20 ± 1.01 | 20.70 ± 5.03 | 15.23 ± 1.43 | 0.121 | 0.001 | 0.100 |
| HTX | 25.49 ± 1.47 | 20.77 ± 1.79 | 18.39 ± 3.59 | ||||
| HVA | Ctrl | 157.29 ± 8.31 Aa | 29.60 ± 4.92 Ab | 37.93 ± 3.41 Ab | 0.019 | <0.001 | 0.011 |
| HTX | 233.09 ± 13.50 Ba | 27.00 ± 2.64 Ab | 47.79 ± 8.27 Ac | ||||
| DOPAC/DA | Ctrl | 0.54 ± 0.08 Aa | 1.46 ± 0.28 Ab | 2.19 ± 0.30 Ab | 0.034 | <0.001 | <0.001 |
| HTX | 1.27 ± 0.05 Ba | 1.72 ± 0.26 Aa | 2.80 ± 0.74 Aa | ||||
| HVA/DA | Ctrl | 5.24 ± 0.80 Aa | 3.14 ± 1.16 Aa | 5.30 ± 0.70 Aa | 0.232 | 0.010 | <0.001 |
| HTX | 11.60 ± 0.45 Ba | 1.67 ± 0.28 Ab | 6.42 ± 1.39 Ac | ||||
| (DOPAC + HVA)/DA | Ctrl | 5.78 ± 0.88 Aa | 4.59 ± 0.87 Aab | 7.48 ± 0.98 Ab | 0.097 | 0.334 | <0.001 |
| HTX | 12.87 ± 0.48 Ba | 3.39 ± 0.41 Ab | 9.22 ± 2.08 Ac | ||||
| DOP total | Ctrl | 265.84 ± 15.60 | 108.75 ± 48.17 | 62.38 ± 4.78 | 0.291 | <0.001 | 0.076 |
| HTX | 278.51 ± 15.05 | 58.30 ± 7.94 | 76.52 ± 11.38 | ||||
| 5-HT | Ctrl | 76.34 ± 3.15 | 243.39 ± 7.37 | 298.03 ± 34.34 | 0.043 | <0.001 | 0.208 |
| HTX | 98.14 ± 6.22 | 257.78 ± 10.50 | 297.83 ± 19.26 | ||||
| 5-HIAA | Ctrl | 57.49 ± 3.00 Aa | 54.33 ± 5.72 Aa | 60.44 ± 3.41 Aa | 0.027 | <0.001 | <0.001 |
| HTX | 91.03 ± 4.83 Ba | 47.61 ± 3.60 Ab | 64.19 ± 5.15 Ac | ||||
| 5-HIAA/5-HT | Ctrl | 0.81 ± 0.06 Aa | 0.22 ± 0.02 Ab | 0.23 ± 0.02 Ab | 0.762 | <0.001 | 0.006 |
| HTX | 1.05 ± 0.06 Ba | 0.19 ± 0.01 Ab | 0.22 ± 0.02 Ab | ||||
| IND total | Ctrl | 133.83 ± 4.85 Aa | 281.18 ± 18.96 Ab | 358.46 ± 34.67 Ab | 0.003 | <0.001 | 0.001 |
| HTX | 189.17 ± 8.47 Ba | 305.40 ± 12.30 Ab | 362.02 ± 23.13 Ab | ||||
| MDA | TAS | GPX | SOD | ||
|---|---|---|---|---|---|
| (pmol/g Tissue) | (µmol/g Tissue) | (U/g Tissue) | (U/g Tissue) | ||
| Prefrontal cortex | Ctrl | 1.49 ± 0.092 | 7.97 ± 0.06 | 1.58 ± 0.10 | 11.09 ± 0.06 |
| HTX | 1.19 ± 0.046 | 7.83 ± 0.07 | 1.89 ± 0.14 | 10.30 ± 0.07 | |
| p-Value | 0.003 | 0.134 | 0.090 | 0.000 | |
| Hippocampus | Ctrl | 43.96 ± 2.58 | 7.43 ± 0.09 | 1.80 ± 0.16 | 9.95 ± 0.15 |
| HTX | 46.20 ± 0.99 | 7.54 ± 0.08 | 1.85 ± 0.16 | 10.00 ± 0.09 | |
| p-Value | 0.424 | 0.383 | 0.838 | 0.771 | |
| CA1 | GD | ||||||
|---|---|---|---|---|---|---|---|
| Cell Count | Area (%) | Mean Size (µm2) | Cell Count | Area (%) | Mean Size (µm2) | ||
| Fetuses | Ctrl | 127.84 ± 8.13 | 35.70 ± 1.31 | 1791.27 ± 275.44 | 79.75 ± 2.02 | 27.00 ± 0.46 | 1496.11 ± 49.92 |
| HTX | 167.74 ± 6.60 | 30.42 ± 0.75 | 823.86 ± 42.61 | 87.25 ± 3.08 | 23.17 ± 0.56 | 1169.11 ± 50.12 | |
| p-Values | <0.001 | 0.001 | 0.001 | 0.039 | <0.001 | <0.001 | |
| 1 month | Ctrl | 163.36 ± 5.52 | 23.20 ± 1.23 | 631.97 ± 51.36 | 58.18 ± 2.25 | 23.42 ± 0.46 | 1772.04 ± 66.80 |
| HTX | 180.78 ± 7.44 | 22.36 ± 0.89 | 555.54 ± 38.25 | 60.30 ± 2.77 | 23.02 ± 0.72 | 1708.03 ± 91.54 | |
| p-Values | 0.068 | 0.579 | 0.236 | 0.557 | 0.642 | 0.578 | |
| 6 months | Ctrl | 119.94 ± 4.58 | 14.01 ± 1.20 | 521.42 ± 58.01 | 28.59 ± 1.67 | 19.75 ± 0.82 | 3110.39 ± 193.77 |
| HTX | 121.59 ± 6.12 | 16.84 ± 1.06 | 629.58 ± 61.29 | 39.53 ± 1.56 | 20.30 ± 1.23 | 2296.83 ± 204.82 | |
| p-Values | 0.831 | 0.087 | 0.209 | <0.001 | 0.709 | 0.007 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeste, N.; Valent, D.; Arroyo, L.; Vázquez-Gómez, M.; García-Contreras, C.; Pumarola, M.; González-Bulnes, A.; Bassols, A. Polyphenols and IUGR Pregnancies: Effects of the Antioxidant Hydroxytyrosol on Brain Neurochemistry and Development in a Porcine Model. Antioxidants 2021, 10, 884. https://doi.org/10.3390/antiox10060884
Yeste N, Valent D, Arroyo L, Vázquez-Gómez M, García-Contreras C, Pumarola M, González-Bulnes A, Bassols A. Polyphenols and IUGR Pregnancies: Effects of the Antioxidant Hydroxytyrosol on Brain Neurochemistry and Development in a Porcine Model. Antioxidants. 2021; 10(6):884. https://doi.org/10.3390/antiox10060884
Chicago/Turabian StyleYeste, Natalia, Daniel Valent, Laura Arroyo, Marta Vázquez-Gómez, Consolación García-Contreras, Martí Pumarola, Antonio González-Bulnes, and Anna Bassols. 2021. "Polyphenols and IUGR Pregnancies: Effects of the Antioxidant Hydroxytyrosol on Brain Neurochemistry and Development in a Porcine Model" Antioxidants 10, no. 6: 884. https://doi.org/10.3390/antiox10060884
APA StyleYeste, N., Valent, D., Arroyo, L., Vázquez-Gómez, M., García-Contreras, C., Pumarola, M., González-Bulnes, A., & Bassols, A. (2021). Polyphenols and IUGR Pregnancies: Effects of the Antioxidant Hydroxytyrosol on Brain Neurochemistry and Development in a Porcine Model. Antioxidants, 10(6), 884. https://doi.org/10.3390/antiox10060884