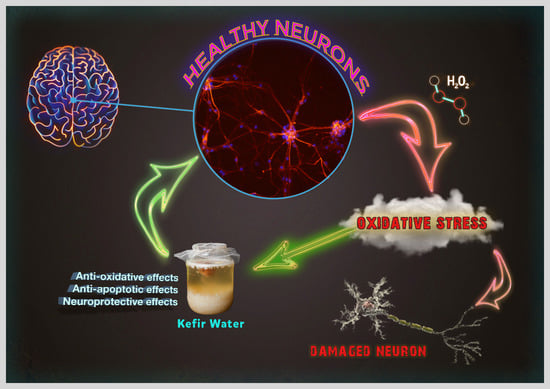
Selected Kefir Water from Malaysia Attenuates Hydrogen Peroxide-Induced Oxidative Stress by Upregulating Endogenous Antioxidant Levels in SH-SY5Y Neuroblastoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation Kefir Water Culture
2.2. Antioxidant Analysis of Kefir Water
2.2.1. Total Phenolic Content
2.2.2. Total Flavonoid Content
2.2.3. 2,2′-Diphenyl-1-picrylhydrazyl Radical Assay
2.2.4. Ferric Reducing Antioxidant Power Assay
2.3. Cell Lines and Cell Culture
2.4. Differentiation of SH-SY5Y Cells
2.5. Immunocytochemistry (ICC) Assay
2.6. MTT Assay
2.7. Acridine Orange and Propidium Iodide (AOPI) Double Staining
2.8. Annexin V-FITC Apoptosis Analysis
2.9. Scanning Electron Microscopy (SEM)
2.10. Transmission Electron Microscopy (TEM)
2.11. Quantitative Real-Time PCR (qPCR) Analysis
2.11.1. RNA Extraction
2.11.2. Primer Design
2.11.3. qPCR Analysis
2.12. Statistical Analysis
3. Results
3.1. In Vitro Antioxidant Analysis
3.2. Differentiation of SH-SY5Y Cells into Neuronal Cells
3.3. Effect of Different Kefir Water Samples on SH-SY5Y Cells Viability
3.4. Effect of Different Kefir Water Samples on H2O2-Induced Cell Death in SH-SY5Y Cells
3.5. Acridine Orange and Propidium Iodide (AO/PI) Double Staining Assay
3.6. Annexin V-FITC Assay
3.7. Scanning Electron Microscopy (SEM)
3.8. Transmission Electron Microscopy (TEM)
3.9. qPCR Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neurodegenerative Diseases. Available online: https://www.niehs.nih.gov/research/supported/health/neurodegenerative/index.cfm (accessed on 30 September 2019).
- Chin, J.H.; Vora, N. The Global Burden of Neurologic Diseases. Neurology 2014, 83, 349–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [Green Version]
- Azmi, N.H.; Ismail, N.; Imam, M.U.; Ismail, M. Ethyl Acetate Extract of Germinated Brown Rice Attenuates Hydrogen Peroxide-Induced Oxidative Stress in Human SH-SY5Y Neuroblastoma Cells: Role of Anti-Apoptotic, pro-Survival and Antioxidant Genes. BMC Complement. Altern. Med. 2013, 13, 177. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-C.; Huang, W.-Y.; Tsai, T.-C.; Hsieh, W.-Y.; Ko, W.-P.; Chang, K.-J.; Chang, T.-M. Supercritical Fluid Extract of Lycium Chinense Miller Root Inhibition of Melanin Production and Its Potential Mechanisms of Action. BMC Complement. Altern. Med. 2014, 14, 208. [Google Scholar] [CrossRef] [Green Version]
- Guyton, K.Z.; Liu, Y.; Gorospe, M.; Xu, Q.; Holbrook, N.J. Activation of Mitogen-Activated Protein Kinase by H2O2. Role in Cell Survival Following Oxidant Injury. J. Biol. Chem. 1996, 271, 4138–4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbert, J.M.; Bono, F.; Savi, P. The Mitogenic Effect of H2O2 for Vascular Smooth Muscle Cells Is Mediated by an Increase of the Affinity of Basic Fibroblast Growth Factor for Its Receptor. FEBS Lett. 1996, 395, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Im, A.-R.; Chae, S.-W.; jun Zhang, G.; Lee, M.-Y. Neuroprotective Effects of Psoralea Corylifolia Linn Seed Extracts on Mitochondrial Dysfunction Induced by 3-Nitropropionic Acid. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Zhan, L.; Liang, L.; Sui, H.; Zheng, L.; Sun, X.; Xie, W. ZiBu PiYin Recipe Prevents Diabetes-Associated Cognitive Decline in Rats: Possible Involvement of Ameliorating Mitochondrial Dysfunction, Insulin Resistance Pathway and Histopathological Changes. BMC Complement. Altern. Med. 2016, 16, 200. [Google Scholar] [CrossRef] [Green Version]
- Sheng, C.; Peng, W.; Xia, Z.; Wang, Y.; Chen, Z.; Su, N.; Wang, Z. The Impact of Ginsenosides on Cognitive Deficits in Experimental Animal Studies of Alzheimer’s Disease: A Systematic Review. BMC Complement. Altern. Med. 2015, 15. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.; Ismail, M.; Imam, M.U.; Azmi, N.H.; Fathy, S.F.; Foo, J.B.; Abu Bakar, M.F. Mechanistic Basis for Protection of Differentiated SH-SY5Y Cells by Oryzanol-Rich Fraction against Hydrogen Peroxide-Induced Neurotoxicity. BMC Complement. Altern. Med. 2014, 14, 467. [Google Scholar] [CrossRef] [Green Version]
- Jaafaru, M.S.; Nordin, N.; Shaari, K.; Rosli, R.; Abdull Razis, A.F. Isothiocyanate from Moringa Oleifera Seeds Mitigates Hydrogen Peroxide-Induced Cytotoxicity and Preserved Morphological Features of Human Neuronal Cells. PLoS ONE 2018, 13, e0196403. [Google Scholar] [CrossRef] [Green Version]
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira Leite, A.M.; Miguel, M.A.L.; Peixoto, R.S.; Rosado, A.S.; Silva, J.T.; Paschoalin, V.M.F. Microbiological, Technological and Therapeutic Properties of Kefir: A Natural Probiotic Beverage. Braz. J. Microbiol. 2013, 44, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Otles, S.; Cagindi, O.; Akcicek, E. Probiotics and Health. Asian Pac. J. Cancer Prev. 2003, 4, 369–372. [Google Scholar]
- Kakisu, E.; Irigoyen, A.; Torre, P.; De Antoni, G.L.; Abraham, A.G. Physicochemical, Microbiological and Sensory Profiles of Fermented Milk Containing Probiotic Strains Isolated from Kefir. J. Dairy Res. 2011, 78, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Sirirat, D.; Jelena, P. Bacterial Inhibition and Antioxidant Activity of Kefir Produced from Thai Jasmine Rice Milk. Biotechnology 2010, 9, 332–337. [Google Scholar] [CrossRef]
- Laureys, D.; Vuyst, L.D. Microbial Species Diversity, Community Dynamics, and Metabolite Kinetics of Water Kefir Fermentation. Appl. Environ. Microbiol. 2014, 80, 2564–2572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verce, M.; De Vuyst, L.; Weckx, S. Shotgun Metagenomics of a Water Kefir Fermentation Ecosystem Reveals a Novel Oenococcus Species. Front. Microbiol. 2019, 10, 479. [Google Scholar] [CrossRef] [PubMed]
- Stadie, J.; Gulitz, A.; Ehrmann, M.A.; Vogel, R.F. Metabolic Activity and Symbiotic Interactions of Lactic Acid Bacteria and Yeasts Isolated from Water Kefir. Food Microbiol. 2013, 35, 92–98. [Google Scholar] [CrossRef]
- Liu, J.-R.; Chen, M.-J.; Lin, C.-W. Antimutagenic and Antioxidant Properties of Milk-Kefir and Soymilk-Kefir. J. Agric. Food Chem. 2005, 53, 2467–2474. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Araújo, T.H.; Schneedorf, J.M.; de Souza Ferreira, C.; de Oliveira Isac Moraes, G.; Coimbra, R.S.; Rodrigues, M.R. A Novel Beer Fermented by Kefir Enhances Anti-Inflammatory and Anti-Ulcerogenic Activities Found Isolated in Its Constituents. J. Funct. Foods 2016, 21, 58–69. [Google Scholar] [CrossRef]
- Güven, A.; Güven, A.; Gülmez, M. The Effect of Kefir on the Activities of GSH-Px, GST, CAT, GSH and LPO Levels in Carbon Tetrachloride-Induced Mice Tissues. J. Vet. Med. B Infect. Dis. Vet. Public Health 2003, 50, 412–416. [Google Scholar] [CrossRef]
- Alsayadi, M.S.M.; Al-jawfi, Y.; Belarbi, M.; Sabri, F.Z. Antioxidant Potency of Water Kefir. J. Microbiol. Biotechnol. Food Sci. 2013, 2, 2444–2447. [Google Scholar]
- Fahmy, H.A.; Ismail, A.F.M. Gastroprotective Effect of Kefir on Ulcer Induced in Irradiated Rats. J. Photochem. Photobiol. B 2015, 144, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Sunarti, S.; Nurliyani, N.; Tyas, A.S.A.; Kristian, S.D.; Prasetyastuti, P. The Influence of Goat Milk and Soybean Milk Kefir on Il-6 and Crp Levels in Diabetic Rats. Rom. J. Diabetes Nutr. Metab. Dis. 2015, 22, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, K.L.; Carvalho, J.C.T.; Schneedorf, J.M. Anti-Inflammatory Properties of Kefir and Its Polysaccharide Extract. Inflammopharmacology 2005, 13, 485–492. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, J.; Yang, X.; Nan, B.; Liu, Y.; Wang, Z. Chemical and Physical Characteristics and Antioxidant Activities of the Exopolysaccharide Produced by Tibetan Kefir Grains during Milk Fermentation. Int. Dairy J. 2015, 43, 15–21. [Google Scholar] [CrossRef]
- Ozcan, T.; Sahin, S.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L. Assessment of Antioxidant Capacity by Method Comparison and Amino Acid Characterisation in Buffalo Milk Kefir. Int. J. Dairy Technol. 2019, 72, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Sabokbar, N.; Khodaiyan, F. Total Phenolic Content and Antioxidant Activities of Pomegranate Juice and Whey Based Novel Beverage Fermented by Kefir Grains. J. Food Sci. Technol. 2016, 53, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Inhibitory Power of Kefir: The Role of Organic Acids. J. Food Prot. 2000, 63, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Guven, M.; Akman, T.; Yener, A.U.; Sehitoglu, M.H.; Yuksel, Y.; Cosar, M. The Neuroprotective Effect of Kefir on Spinal Cord Ischemia/Reperfusion Injury in Rats. J. Korean Neurosurg. Soc. 2015, 57, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Talib, N.; Mohamad, N.E.; Yeap, S.K.; Hussin, Y.; Aziz, M.N.M.; Masarudin, M.J.; Sharifuddin, S.A.; Hui, Y.W.; Ho, C.L.; Alitheen, N.B. Isolation and Characterization of Lactobacillus Spp. from Kefir Samples in Malaysia. Molecules 2019, 24, 2606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Li, C.; He, Z.; Pan, F.; Pan, S.; Wang, Y. Probiotic Properties and Cellular Antioxidant Activity of Lactobacillus Plantarum MA2 Isolated from Tibetan Kefir Grains. Probiotics Antimicrob. Proteins 2018, 10, 523–533. [Google Scholar] [CrossRef]
- De Oliveira Coelho, B.; Fiorda-Mello, F.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Rakshit, S.K.; de Carvalho, J.C.; Soccol, C.R. In Vitro Probiotic Properties and DNA Protection Activity of Yeast and Lactic Acid Bacteria Isolated from A Honey-Based Kefir Beverage. Foods 2019, 8, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant Activity, Total Phenolic and Total Flavonoid Contents of Whole Plant Extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef] [Green Version]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Lopes, F.M.; Schröder, R.; da Frota Júnior, M.L.C.; Zanotto-Filho, A.; Müller, C.B.; Pires, A.S.; Meurer, R.T.; Colpo, G.D.; Gelain, D.P.; Kapczinski, F.; et al. Comparison between Proliferative and Neuron-like SH-SY5Y Cells as an in Vitro Model for Parkinson Disease Studies. Brain Res. 2010, 1337, 85–94. [Google Scholar] [CrossRef]
- Mantzourani, I.; Chondrou, P.; Bontsidis, C.; Karolidou, K.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Galanis, A.; Plessas, S. Assessment of the Probiotic Potential of Lactic Acid Bacteria Isolated from Kefir Grains: Evaluation of Adhesion and Antiproliferative Properties in in Vitro Experimental Systems. Ann. Microbiol. 2019, 69, 751–763. [Google Scholar] [CrossRef]
- Lin, X.; Xia, Y.; Wang, G.; Yang, Y.; Xiong, Z.; Lv, F.; Zhou, W.; Ai, L. Lactic Acid Bacteria With Antioxidant Activities Alleviating Oxidized Oil Induced Hepatic Injury in Mice. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, L.; Duan, Y.; Yang, X. Antioxidant Activity of Lactobacillus plantarum JM113 in Vitro and Its Protective Effect on Broiler Chickens Challenged with Deoxynivalenol. J. Anim. Sci. 2017, 95, 837–846. [Google Scholar] [CrossRef]
- Xing, J.; Wang, G.; Zhang, Q.; Liu, X.; Gu, Z.; Zhang, H.; Chen, Y.Q.; Chen, W. Determining Antioxidant Activities of Lactobacilli Cell-Free Supernatants by Cellular Antioxidant Assay: A Comparison with Traditional Methods. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Kachouri, F.; Ksontini, H.; Kraiem, M.; Setti, K.; Mechmeche, M.; Hamdi, M. Involvement of Antioxidant Activity of Lactobacillus Plantarum on Functional Properties of Olive Phenolic Compounds. J. Food Sci. Technol. 2015, 52, 7924–7933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, Y.-T.; Lau, W.K.-W.; Yu, M.-S.; Lai, C.S.-W.; Yeung, S.-C.; So, K.-F.; Chang, R.C.-C. Effects of All-Trans-Retinoic Acid on Human SH-SY5Y Neuroblastoma as in Vitro Model in Neurotoxicity Research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Korecka, J.A.; van Kesteren, R.E.; Blaas, E.; Spitzer, S.O.; Kamstra, J.H.; Smit, A.B.; Swaab, D.F.; Verhaagen, J.; Bossers, K. Phenotypic Characterization of Retinoic Acid Differentiated SH-SY5Y Cells by Transcriptional Profiling. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwane, S.; Durack, E.; Kiely, P.A. Optimising Parameters for the Differentiation of SH-SY5Y Cells to Study Cell Adhesion and Cell Migration. BMC Res. Notes 2013, 6, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. JoVE 2016, 53193. [Google Scholar] [CrossRef]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Ceña, V.; Gallego, C.; Comella, J.X. Sequential Treatment of SH-SY5Y Cells with Retinoic Acid and Brain-Derived Neurotrophic Factor Gives Rise to Fully Differentiated, Neurotrophic Factor-Dependent, Human Neuron-like Cells. J. Neurochem. 2000, 75, 991–1003. [Google Scholar] [CrossRef]
- Gimenez-Cassina, A.; Lim, F.; Diaz-Nido, J. Differentiation of a Human Neuroblastoma into Neuron-like Cells Increases Their Susceptibility to Transduction by Herpesviral Vectors. J. Neurosci. Res. 2006, 84, 755–767. [Google Scholar] [CrossRef]
- Biedler, J.L.; Helson, L.; Spengler, B.A. Morphology and Growth, Tumorigenicity, and Cytogenetics of Human Neuroblastoma Cells in Continuous Culture. Cancer Res. 1973, 33, 2643–2652. [Google Scholar]
- Christensen, J.; Steain, M.; Slobedman, B.; Abendroth, A. Differentiated Neuroblastoma Cells Provide a Highly Efficient Model for Studies of Productive Varicella-Zoster Virus Infection of Neuronal Cells. J. Virol. 2011, 85, 8436–8442. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, Y.; Du, W.; Liu, W.; Liu, F.; Zhang, L.; Zhang, M.; Hou, M.; Liu, K.; Zhang, S.; et al. Wnt1 Inhibits Hydrogen Peroxide-Induced Apoptosis in Mouse Cardiac Stem Cells. PLoS ONE 2013, 8, e58883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobwala, S.; Fan, W.; Hines, C.J.; Folk, W.R.; Ercal, N. Antioxidant Potential of Sutherlandia Frutescens and Its Protective Effects against Oxidative Stress in Various Cell Cultures. BMC Complement. Altern. Med. 2014, 14, 271. [Google Scholar] [CrossRef] [Green Version]
- Isachenko, V.; Todorov, P.; Isachenko, E.; Rahimi, G.; Tchorbanov, A.; Mihaylova, N.; Manoylov, I.; Mallmann, P.; Merzenich, M. Long-Time Cooling before Cryopreservation Decreased Translocation of Phosphatidylserine (Ptd-L-Ser) in Human Ovarian Tissue. PLoS ONE 2015, 10, e0129108. [Google Scholar] [CrossRef]
- Takeda, M.; Shirato, I.; Kobayashi, M.; Endou, H. Hydrogen Peroxide Induces Necrosis, Apoptosis, Oncosis and Apoptotic Oncosis of Mouse Terminal Proximal Straight Tubule Cells. Nephron 1999, 81, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kang, J.; Fu, C. The Independence of and Associations among Apoptosis, Autophagy, and Necrosis. Signal Transduct. Target. Ther. 2018, 3, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, S.L.; Cookson, B.T. Apoptosis, Pyroptosis, and Necrosis: Mechanistic Description of Dead and Dying Eukaryotic Cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, H.; Ding, K.; Xu, J. FTY720 Induces Autophagy-Related Apoptosis and Necroptosis in Human Glioblastoma Cells. Toxicol. Lett. 2015, 236, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, S.; Nakanomyo, I.; Kudo-Sakamoto, Y.; Akazawa, H.; Komuro, I.; Shimizu, S. Identification of a Novel Compound That Inhibits Both Mitochondria-Mediated Necrosis and Apoptosis. Biochem. Biophys. Res. Commun. 2015, 467, 1006–1011. [Google Scholar] [CrossRef]
- Wang, X.; Ryter, S.W.; Dai, C.; Tang, Z.-L.; Watkins, S.C.; Yin, X.-M.; Song, R.; Choi, A.M.K. Necrotic Cell Death in Response to Oxidant Stress Involves the Activation of the Apoptogenic Caspase-8/Bid Pathway. J. Biol. Chem. 2003, 278, 29184–29191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leist, M.; Jäättelä, M. Four Deaths and a Funeral: From Caspases to Alternative Mechanisms. Nat. Rev. Mol. Cell Biol. 2001, 2, 589–598. [Google Scholar] [CrossRef]
- Szymański, J.; Janikiewicz, J.; Michalska, B.; Patalas-Krawczyk, P.; Perrone, M.; Ziółkowski, W.; Duszyński, J.; Pinton, P.; Dobrzyń, A.; Więckowski, M.R. Interaction of Mitochondria with the Endoplasmic Reticulum and Plasma Membrane in Calcium Homeostasis, Lipid Trafficking and Mitochondrial Structure. Int. J. Mol. Sci. 2017, 18, 1576. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The Effects of Bioactive Compounds from Plant Foods on Mitochondrial Function: A Focus on Apoptotic Mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Chen, L.; Yu, F.; Li, X.; Dan, T.; Zhao, J.; Zhou, S. Polyphyllin I Induces G2/M Phase Arrest and Apoptosis in U251 Human Glioma Cells via Mitochondrial Dysfunction and the JNK Signaling Pathway. Acta Biochim. Biophys. Sin. 2017, 49, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiewe, T.; Pützer, B.M. P73 in Apoptosis. Apoptosis 2001, 6, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Castellino, R.C.; De Bortoli, M.; Lin, L.L.; Skapura, D.G.; Rajan, J.A.; Adesina, A.M.; Perlaky, L.; Irwin, M.S.; Kim, J.Y. Overexpressed TP73 Induces Apoptosis in Medulloblastoma. BMC Cancer 2007, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Klein, J.A.; Ackerman, S.L. Oxidative Stress, Cell Cycle, and Neurodegeneration. J. Clin. Investig. 2003, 111, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma Membrane Changes during Programmed Cell Deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Raguenez, G.; Desire, L.; Lantrua, V.; Courtois, Y. BCL-2 Is Upregulated in Human SH-SY5Y Neuroblastoma Cells Differentiated by Overexpression of Fibroblast Growth Factor 1. Biochem. Biophys. Res. Commun. 1999, 258, 745–751. [Google Scholar] [CrossRef]








| Gene | Accession Number | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| SOD2 | NM_000636 | AAACTGAGAGCAAAGAATGGAG | CCACAAGCACAGAAATAAAGG |
| Catalase | NM_001752 | GGTAACCCAGTAGGAGACAAAC | CGAGATCCCAGTTACCATCTTTC |
| Tp73 | NM_005427.4 | AGCAGCCCATCAAGGAGGAGTT | TCCTGAGGCAGTTTTGGACACA |
| Bax | NM_001291428 | CAAGAAGCTGAGCGAGTGT | CAGTTGAAGTTGCCGTCAGA |
| Bcl-2 | NM_000633 | TTGACAGAGGATCATGCTGTACTT | ATCTTTATTTCATGAGGCACGTT |
| ACTB | NM_001101 | AGAGCTACGAGCTGCCTGAC | AGCACTGTGTTGGCGTACAG |
| GAPDH | NM_002046 | GGATTTGGTCGTATTGGGC | TGGAAGATGGTGATGGGATT |
| Kefir Samples | TPC (µg GAE/µL Kefir Sample) # | TFC (µg CAT eq/µL Kefir Sample) # | DPPH Free Radical Scavenging Assay (mg/mL) * | FRAP (mM FRAP eq/50 µL Kefir Sample) ** |
|---|---|---|---|---|
| Kefir A | 1.11 ± 0.64 | 1.01 ± 0.01 | 0.76 ± 0.32 | 18.32 ± 0.10 |
| Kefir B | 1.96 ± 0.54 *** | 1.09 ± 0.02 | 0.45 ± 0.06 | 19.68 ± 0.11 |
| Kefir C | 0.68 ± 0.28 | 0.92 ± 0.01 | 0.80 ± 0.01 | 15.25 ± 0.49 |
| Kefir D | 1.43 ± 0.37 | 0.96 ± 0.01 | 0.58 ± 0.03 | 17.87 ± 0.38 |
| Kefir E | 1.14 ± 0.12 | 0.98 ± 0.01 | 0.49 ± 0.16 | 18.11 ± 0.23 |
| Kefir F | ND | 0.91 ± 0.01 | 0.68 ± 0.16 | 17.24 ± 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.R.; Yeap, S.K.; Lee, H.C.; Mohamad, N.E.; Nazirul Mubin Aziz, M.; Khalid, M.; Masarudin, M.J.; Leow, A.T.C.; Abdullah, J.O.; Alitheen, N.B. Selected Kefir Water from Malaysia Attenuates Hydrogen Peroxide-Induced Oxidative Stress by Upregulating Endogenous Antioxidant Levels in SH-SY5Y Neuroblastoma Cells. Antioxidants 2021, 10, 940. https://doi.org/10.3390/antiox10060940
Kumar MR, Yeap SK, Lee HC, Mohamad NE, Nazirul Mubin Aziz M, Khalid M, Masarudin MJ, Leow ATC, Abdullah JO, Alitheen NB. Selected Kefir Water from Malaysia Attenuates Hydrogen Peroxide-Induced Oxidative Stress by Upregulating Endogenous Antioxidant Levels in SH-SY5Y Neuroblastoma Cells. Antioxidants. 2021; 10(6):940. https://doi.org/10.3390/antiox10060940
Chicago/Turabian StyleKumar, Muganti Rajah, Swee Keong Yeap, Han Chung Lee, Nurul Elyani Mohamad, Muhammad Nazirul Mubin Aziz, Melati Khalid, Mas Jaffri Masarudin, Adam Thean Chor Leow, Janna Ong Abdullah, and Noorjahan Banu Alitheen. 2021. "Selected Kefir Water from Malaysia Attenuates Hydrogen Peroxide-Induced Oxidative Stress by Upregulating Endogenous Antioxidant Levels in SH-SY5Y Neuroblastoma Cells" Antioxidants 10, no. 6: 940. https://doi.org/10.3390/antiox10060940
APA StyleKumar, M. R., Yeap, S. K., Lee, H. C., Mohamad, N. E., Nazirul Mubin Aziz, M., Khalid, M., Masarudin, M. J., Leow, A. T. C., Abdullah, J. O., & Alitheen, N. B. (2021). Selected Kefir Water from Malaysia Attenuates Hydrogen Peroxide-Induced Oxidative Stress by Upregulating Endogenous Antioxidant Levels in SH-SY5Y Neuroblastoma Cells. Antioxidants, 10(6), 940. https://doi.org/10.3390/antiox10060940