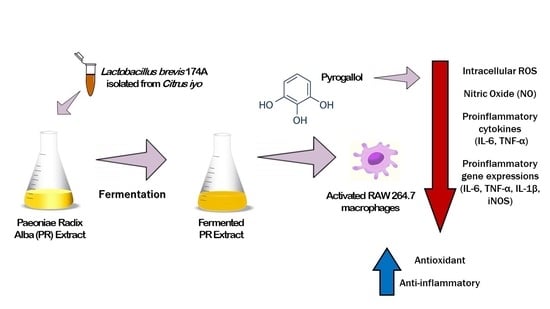
Anti-Oxidant and Anti-Inflammatory Substance Generated Newly in Paeoniae Radix Alba Extract Fermented with Plant-Derived Lactobacillus brevis 174A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria Culture and Fermentation Conditions
2.2. Measurement of Total Phenolic Content (TPC)
2.3. Cell Culture and Treatment
2.4. Cell Viability Assay
2.5. Measurement of Intracellular ROS Levels
2.6. Measurement of NO Production
2.7. Inflammatory Cytokine Determination
2.8. RNA Extraction and qRT-PCR Analysis
2.9. HPLC Analysis, Extraction, and Identification of a Newly Produced Compound in the Fermented Extract
2.10. Statistical Analyses
3. Results and Discussion
3.1. Simultaneous Increase in Total Phenolic Content and Bioactivity in the Fermented PR
3.2. Significant Decrease in Inflammatory Gene Expression and Release of NO and TNF-α by Fermented PR Extract
3.3. HPLC Analysis and Identification of a Newly Produced Compound in the Fermented Extract
3.4. Pyrogallol as a Sole Bioactive Compound
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leroy, F.; De Vuyst, L. Fermented food in the context of a healthy diet. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pang, H.; Zhang, H.; Cai, Y. Biodiversity of Lactic Acid Bacteria. In Lactic Acid Bacteria; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Noda, M.; Danshiitsoodol, N.; Inoue, Y.; Okamoto, T.; Sultana, N.; Sugiyama, M. Antibiotic susceptibility of plant-derived lactic acid bacteria conferring health benefits to human. J. Antibiot. 2019, 72, 834–842. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Nomura, K.; Oku, H.; Sugiyama, M. Improvement of constipation and liver function by plant-derived lactic acid bacteria: A double-blind, randomized trial. Nutrition 2010, 26, 367–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasutake, T.; Kumagai, T.; Inoue, A.; Kobayashi, K.; Noda, M.; Orikawa, A.; Matoba, Y.; Sugiyama, M. Characterization of the LP28 strain-specific exopolysaccharide biosynthetic gene cluster found in the whole circular genome of Pediococcus pentosaceus. Biochem. Biophys. Rep. 2016, 5, 266–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, M.; Shiraga, M.; Kumagai, T.; Danshiitsoodol, N.; Sugiyama, M. Characterization of the SN35N Strain-Specific Exopolysaccharide Encoded in the Whole Circular Genome of a Plant-Derived Lactobacillus plantarum. Biol. Pharm. Bull. 2018, 41, 536–545. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Higashikawa, F.; Noda, M.; Kawamura, Y.; Matoba, Y.; Kumagai, T.; Sugiyama, M. The Obesity and Fatty Liver are Reduced by Plant-Derived Pediococcus pentosaceus LP28 in High Fat Diet-Induced Obese Mice. PLoS ONE 2012, 7, e30696. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Higashikawa, F.; Noda, M.; Zhao, X.; Matoba, Y.; Kumagai, T.; Sugiyama, M. Establishment of an in Vitro Peyer’s Patch Cell Culture System Correlative to in Vivo Study Using Intestine and Screening of Lactic Acid Bacteria Enhancing Intestinal Immunity. Biol. Pharm. Bull. 2010, 33, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, T.; Sugimoto, S.; Noda, M.; Yokooji, T.; Danshiitsoodol, N.; Higashikawa, F.; Sugiyama, M. Interleukin-8 Release Inhibitors Generated by Fermentation of Artemisia princeps Pampanini Herb Extract with Lactobacillus plantarum SN13T. Front. Microbiol. 2020, 11, 1159. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Novel Fermented Fruit and Vegetable-Based Products. In Novel Food Fermentation Technologies; Springer: Cham, Germany, 2016. [Google Scholar] [CrossRef]
- Hussain, A.; Bose, S.; Wang, J.-H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Ehe, D.-Y.; Edai, S.-M. Anti-Inflammatory and Immunomodulatory Effects of Paeonia lactiflora Pall., a Traditional Chinese Herbal Medicine. Front. Pharmacol. 2011, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Kwon, Y.S.; Son, K.H.; Kim, H.P.; Heo, M.Y. Antioxidative constituents from Paeonia lactiflora. Arch. Pharm. Res. 2005, 28, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Shu, Y.-Z.; Matsuda, Y.; Hattori, M.; Namba, T.; Kobashi, K. Metabolism of paeoniflorin and related compounds by human intestinal bacteria. IV. Formation and structures of adducts of a metabolic intermediate with sulfhydryl compounds by Lactobacillus brevis. Chem. Pharm. Bull. 1988, 36, 3043–3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.-B.; Zhao, Z.-X.; Peng, R.; Pan, L.-B.; Fu, J.; Ma, S.-R.; Han, P.; Cong, L.; Zhang, Z.-W.; Sun, L.-X.; et al. Gut Microbiota-Based Pharmacokinetics and the Antidepressant Mechanism of Paeoniflorin. Front. Pharmacol. 2019, 10, 268. [Google Scholar] [CrossRef] [Green Version]
- Hattori, M.; Shu, Y.; Shimizu, M.; Hayashi, T.; Morita, N.; Kobashi, K.; Xu, G.; Namba, T. Metabolism of paeoniflorin and related compounds by human intestinal bacteria. Chem. Pharm. Bull. 1985, 33, 3838–3846. [Google Scholar] [CrossRef] [Green Version]
- Noda, M.; Miyauchi, R.; Danshiitsoodol, N.; Higashikawa, F.; Kumagai, T.; Matoba, Y.; Sugiyama, M. Characterization and Mutational Analysis of a Two-Polypeptide Bacteriocin Produced by Citrus Iyo-Derived Lactobacillus brevis 174A. Biol. Pharm. Bull. 2015, 38, 1902–1909. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Li, Z. Neuroprotective effect of paeoniflorin on H2O2-induced apoptosis in PC12 cells by modulation of reactive oxygen species and the inflammatory response. Exp. Ther. Med. 2015, 9, 1768–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.H.; Ma, J.N.; Park, J.H.; Jung, H.W.; Park, Y. Anti-inflammatory and antioxidant effects of MOK, a polyherbal extract, on lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Mol. Med. 2018, 43, 26–36. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, R.; Zhang, Y.; Yang, Y.; Sun, X.; Zhang, Q.; Yang, N. Biotransformation of phenolics and metabolites and the change in antioxidant activity in kiwifruit induced by Lactobacillus plantarum fermentation. J. Sci. Food Agric. 2020, 100, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Jimïnez, N.; Curiel, J.A.; Reverï, N.I.; Rivas, B.D.L.; Muïoz, R. Uncovering the Lactobacillus plantarum WCFS1 Gallate Decarboxylase Involved in Tannin Degradation. Appl. Environ. Microbiol. 2013, 79, 4253–4263. [Google Scholar] [CrossRef] [Green Version]
- Sabokbar, N.; Khodaiyan, F. Total phenolic content and antioxidant activities of pomegranate juice and whey based novel beverage fermented by kefir grains. J. Food Sci. Technol. 2016, 53, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Suryanarayana, L.C.; Chandrashekara, K.A.; Krishnan, P.; Kush, A.; Ravikumar, P. Lactobacillus plantarum mediated fermentation of Psidium guajava L. fruit extract. J. Biosci. Bioeng. 2015, 119, 430–432. [Google Scholar] [CrossRef]
- Adebo, O.A.; Medina-Meza, I.G. Impact of Fermentation on the Phenolic Compounds and Antioxidant Activity of Whole Cereal Grains: A Mini Review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus plantarum ATCC14917. Molecules 2018, 24, 51. [Google Scholar] [CrossRef] [Green Version]
- Muniandy, K.; Gothai, S.; Badran, K.M.H.; Kumar, S.S.; Esa, N.M.; Arulselvan, P. Suppression of Proinflammatory Cytokines and Mediators in LPS-Induced RAW 264.7 Macrophages by Stem Extract of Alternanthera sessilis via the Inhibition of the NF-κB Pathway. J. Immunol. Res. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.D.; Ha, B.J. Paeoniflorin protects RAW 264.7 macrophages from LPS-induced cytotoxicity and genotoxicity. Toxicol. Vitr. 2009, 23, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Cavoski, I.; Thligene, N.; Vincentini, O.; De Angelis, M.; Silano, M.; Gobbetti, M.; Di Cagno, R. Correction: Lactic Acid Fermentation of Cactus Cladodes (Opuntia ficus-indica L.) Generates Flavonoid Derivatives with Antioxidant and Anti-Inflammatory Properties. PLoS ONE 2016, 11, e0155156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Shah, N.P. Lactic acid bacterial fermentation modified phenolic composition in tea extracts and enhanced their antioxidant activity and cellular uptake of phenolic compounds following in vitro digestion. J. Funct. Foods 2016, 20, 182–194. [Google Scholar] [CrossRef]
- Sun, S.; Zhu, L.; Hu, Y.; Liu, Y. Studies on the metabolism of paeoniflorin in human intestinal microflora by high performance liquid chromatography/electrospray ionization/Fourier transform ion cyclotron resonance mass spectrometry and quadrupole time-of-flight mass spectrometry. J. Chromatogr. B 2018, 1085, 63–71. [Google Scholar] [CrossRef]
- Zhao, Z.-X.; Fu, J.; Ma, S.-R.; Peng, R.; Yu, J.-B.; Cong, L.; Pan, L.-B.; Zhang, Z.-G.; Tian, H.; Che, C.-T.; et al. Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics 2018, 8, 5945–5959. [Google Scholar] [CrossRef]
- Yoshida, H.; Yamada, H. Microbial Production of Pyrogallol through Decarboxylation of Gallic Acid. Agric. Biol. Chem. 1985, 49, 659–663. [Google Scholar] [CrossRef]
- Zhang, M.; Otake, K.; Miyauchi, Y.; Yagi, M.; Yonei, Y.; Miyakawa, T.; Tanokura, M. Comprehensive NMR analysis of two kinds of post-fermented tea and their anti-glycation activities in vitro. Food Chem. 2019, 277, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Torres, M.; Santamaría, L.; Cabrera-Rubio, R.; Plaza-Vinuesa, L.; Crispie, F.; Rivas, B.D.L.; Cotter, P.; Muñoz, R. A Diverse Range of Human Gut Bacteria Have the Potential to Metabolize the Dietary Component Gallic Acid. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemec, M.J.; Kim, H.; Marciante, A.B.; Barnes, R.C.; Talcott, S.T.; Mertens-Talcott, S.U. Pyrogallol, an absorbable microbial gallotannins-metabolite and mango polyphenols (Mangifera indica L.) suppress breast cancer ductal carcinoma in situ proliferation in vitro. Food Funct. 2016, 7, 3825–3833. [Google Scholar] [CrossRef] [PubMed]
- Nicolis, E.; Lampronti, I.; Dechecchi, M.C.; Borgatti, M.; Tamanini, A.; Bianchi, N.; Bezzerri, V.; Mancini, I.; Giri, M.G.; Rizzotti, P.; et al. Pyrogallol, an active compound from the medicinal plant Emblica officinalis, regulates expression of pro-inflammatory genes in bronchial epithelial cells. Int. Immunopharmacol. 2008, 8, 1672–1680. [Google Scholar] [CrossRef]
- Chicas, M.C.; Fang, C.; Talcott, S.; Talcott, S. Microbial Metabolites of Gallotannins Suppress Inflammation in RAW 264.7 Macrophages Through the Modulation of the AMPK/NF-kb Axis. Curr. Dev. Nutr. 2020, 4, 375. [Google Scholar] [CrossRef]
- Na Kim, H.; Kim, J.D.; Bin Park, S.; Son, H.-J.; Park, G.H.; Eo, H.J.; Kim, H.-S.; Jeong, J.B. Anti-inflammatory activity of the extracts from Rodgersia podophylla leaves through activation of Nrf2/HO-1 pathway, and inhibition of NF-κB and MAPKs pathway in mouse macrophage cells. Inflamm. Res. 2020, 69, 233–244. [Google Scholar] [CrossRef]
- Nakano, T.; Ikeda, M.; Wakugawa, T.; Kashiwada, Y.; Kaminuma, O.; Kitamura, N.; Yabumoto, M.; Fujino, H.; Kitamura, Y.; Fukui, H.; et al. Identification of pyrogallol from Awa-tea as an anti-allergic compound that suppresses nasal symptoms and IL-9 gene expression. J. Med. Investig. 2020, 67, 289–297. [Google Scholar] [CrossRef]
- Khan, M.T.; Lampronti, I.; Martello, D.; Bianchi, N.; Jabbar, S.; Choudhuri, M.S.; Datta, B.K.; Gambari, R. Identification of pyrogallol as an antiproliferative compound present in extracts from the medicinal plant Emblica officinalis: Effects on in vitro cell growth of human tumor cell lines. Int. J. Oncol. 2002, 21, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Fukuyama, Y.; Nishiwaki, H.; Masuda, A.; Masuda, T. Conversion to purpurogallin, a key step in the mechanism of the potent xanthine oxidase inhibitory activity of pyrogallol. Free. Radic. Biol. Med. 2017, 106, 228–235. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakya, S.; Danshiitsoodol, N.; Sugimoto, S.; Noda, M.; Sugiyama, M. Anti-Oxidant and Anti-Inflammatory Substance Generated Newly in Paeoniae Radix Alba Extract Fermented with Plant-Derived Lactobacillus brevis 174A. Antioxidants 2021, 10, 1071. https://doi.org/10.3390/antiox10071071
Shakya S, Danshiitsoodol N, Sugimoto S, Noda M, Sugiyama M. Anti-Oxidant and Anti-Inflammatory Substance Generated Newly in Paeoniae Radix Alba Extract Fermented with Plant-Derived Lactobacillus brevis 174A. Antioxidants. 2021; 10(7):1071. https://doi.org/10.3390/antiox10071071
Chicago/Turabian StyleShakya, Shrijana, Narandalai Danshiitsoodol, Sachiko Sugimoto, Masafumi Noda, and Masanori Sugiyama. 2021. "Anti-Oxidant and Anti-Inflammatory Substance Generated Newly in Paeoniae Radix Alba Extract Fermented with Plant-Derived Lactobacillus brevis 174A" Antioxidants 10, no. 7: 1071. https://doi.org/10.3390/antiox10071071
APA StyleShakya, S., Danshiitsoodol, N., Sugimoto, S., Noda, M., & Sugiyama, M. (2021). Anti-Oxidant and Anti-Inflammatory Substance Generated Newly in Paeoniae Radix Alba Extract Fermented with Plant-Derived Lactobacillus brevis 174A. Antioxidants, 10(7), 1071. https://doi.org/10.3390/antiox10071071