Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry
Abstract
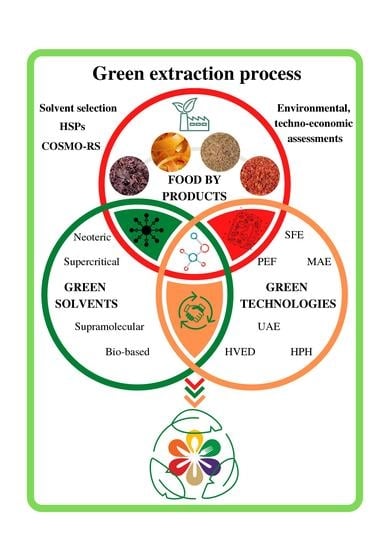
:1. Introduction
- Neoteric solvents;
- Supercritical fluids;
- Bio-based solvents;
- Supramolecular solvents.
2. Emerging Green Technologies and Involved Mechanisms of Cell Disintegration
2.1. Supercritical Fluid Extraction (SFE)
2.2. Microwave-Assisted Extraction (MAE)
2.3. Ultrasound-Assisted Extraction (UAE)
2.4. Pulsed Electric Fields (PEF)
2.5. High Voltage Electrical Discharge (HVED)
2.6. High-Pressure Homogenization (HPH)
- Standard homogenization for pressures between 0 and 50 MPa;
- High-pressure homogenization (HPH) for pressures between 50 and 300 MPa;
- Homogenization at very high pressure (UHPH) for pressures equal to or greater than 400 MPa.
- improving the extraction capacity of intracellular structural components;
- improving the technological functionality of bioactive compounds.
3. Green Extraction Process: Synergism between Solvents and Technology
3.1. Neoteric Solvents
3.1.1. Ionic Liquids
3.1.2. Deep Eutectic Solvent (DES)
3.2. Supercritical Fluids (SCFs)
3.2.1. Water
3.2.2. Carbon Dioxide
3.3. Supramolecular Solvents (SUPRAS)
3.4. Bio-Based Solvents
3.4.1. Ethanol
3.4.2. Glycerol and D-limonene
3.4.3. Water
4. Green Solvents Selection
4.1. Physical Properties
4.2. Environmental Assessment
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Glavič, P.; Pintarič, Z.N.; Bogataj, M. Process Design and Sustainable Development—A European Perspective. Processes 2021, 9, 148. [Google Scholar] [CrossRef]
- Del Pilar Sánchez-Camargo, A.; Pleite, N.; Herrero, M.; Cifuentes, A.; Ibáñez, E.; Gilbert-López, B. New Approaches for the Selective Extraction of Bioactive Compounds Employing Biobased Solvents and Pressurized Green Processes. J. Supercrit. Fluids 2017, 128, 112–120. [Google Scholar] [CrossRef]
- Aissou, M.; Chemat-Djenni, Z.; Yara-Varón, E.; Fabiano-Tixier, A.S.; Chemat, F. Limonene as an Agro-Chemical Building Block for the Synthesis and Extraction of Bioactive Compounds. Comptes Rendus Chim. 2017, 20, 346–358. [Google Scholar] [CrossRef]
- Das, S.; Mondal, A.; Balasubramanian, S. Recent Advances in Modeling Green Solvents. Curr. Opin. Green Sustain. Chem. 2017, 5, 37–43. [Google Scholar] [CrossRef]
- McDaniel, J.G.; Yethiraj, A. Understanding the Properties of Ionic Liquids: Electrostatics, Structure Factors, and Their Sum Rules. J. Phys. Chem. B 2019, 123, 3499–3512. [Google Scholar] [CrossRef]
- Wu, M.; Ma, H.; Ma, Z.; Jin, Y.; Chen, C.; Guo, X.; Qiao, Y.; Pedersen, C.M.; Hou, X.; Wang, Y. Deep Eutectic Solvents: Green Solvents and Catalysts for the Preparation of Pyrazine Derivatives by Self-Condensation of d-Glucosamine. ACS Sustain. Chem. Eng. 2018, 6, 9434–9441. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart Advanced Solvents for Bioactive Compounds Recovery from Agri-Food By-Products: A Review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Jesus, S.P.; Meireles, M.A.A. Supercritical Fluid Extraction: A Global Perspective of the Fundamental Concepts of this Eco-Friendly Extraction Technique; Springer: Berlin/Heidelberg, Germany, 2014; pp. 39–72. [Google Scholar]
- Sarris, D.; Papanikolaou, S. Biotechnological Production of Ethanol: Biochemistry, Processes and Technologies. Eng. Life Sci. 2016, 16, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Makris, D.P.; Lalas, S. Glycerol and Glycerol-Based Deep Eutectic Mixtures as Emerging Green Solvents for Polyphenol Extraction: The Evidence So Far. Molecules 2020, 25, 5842. [Google Scholar] [CrossRef]
- Ballesteros-Gómez, A.; Sicilia, M.D.; Rubio, S. Supramolecular Solvents in the Extraction of Organic Compounds: A review. Anal. Chim. Acta 2010, 677, 108–130. [Google Scholar] [CrossRef]
- Clark, J.H.; Deswarte, F.E.I.; Farmer, T.J. The Integration of Green Chemistry into Future Biorefineries. Biofuels Bioprod. Biorefining 2009, 3, 72–90. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citruswastes-A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, M.; Kravchuk, O.; Skouroumounis, G.K.; Taylor, D.K. Response Surface Parallel Optimization of Extraction of Total Phenolics from Separate White and Red Grape Skin Mixtures with Microwave-Assisted and Conventional Thermal Methods. J. Clean. Prod. 2020, 251, 119563. [Google Scholar] [CrossRef]
- Plazzotta, S.; Ibarz, R.; Manzocco, L.; Martín-Belloso, O. Modelling the Recovery of Biocompounds from Peach Waste Assisted by Pulsed Electric Fields or Thermal Treatment. J. Food Eng. 2021, 290, 110196. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Content of Bioactive Compounds in the Peach Kernels and Their Antioxidant, Anti-Hyperglycemic, Anti-Aging Properties. Eur. Food Res. Technol. 2019, 245, 1123–1136. [Google Scholar] [CrossRef] [Green Version]
- Rico, X.; Gullón, B.; Alonso, J.L.; Yáñez, R. Recovery of High Value-Added Compounds from Pineapple, Melon, Watermelon and Pumpkin Processing By-Products: An Overview. Food Res. Int. 2020, 132, 109086. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Maximising Recovery of Phenolic Compounds and Antioxidant Properties from Banana Peel Using Microwave Assisted Extraction and Water. J. Food Sci. Technol. 2019, 56, 1360–1370. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed Electric Field Assisted Extraction of Nutritionally Valuable Compounds from Microalgae Nannochloropsis spp. Using the Binary Mixture of Organic Solvents and Water. Innov. Food Sci. Emerg. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Niu, D.; Ren, E.F.; Li, J.; Zeng, X.A.; Li, S.L. Effects of Pulsed Electric Field-Assisted Treatment on the Extraction, Antioxidant Activity and Structure of Naringin. Sep. Purif. Technol. 2021, 265, 118480. [Google Scholar] [CrossRef]
- Gonçalves Rodrigues, L.G.; Mazzutti, S.; Vitali, L.; Micke, G.A.; Ferreira, S.R.S. Recovery of Bioactive Phenolic Compounds from Papaya Seeds Agroindustrial Residue Using Subcritical Water Extraction. Biocatal. Agric. Biotechnol. 2019, 22, 101367. [Google Scholar] [CrossRef]
- Boukroufa, M.; Boutekedjiret, C.; Petigny, L.; Rakotomanomana, N.; Chemat, F. Biorefinery of Orange Peels Waste: A New Concept Based on Integrated Green and Solvent Free Extraction Processes Using Ultrasound and Microwave Techniques To Obtain Essential Oil, Polyphenols and Pectin. Ultrason. Sonochem. 2015, 24, 72–79. [Google Scholar] [CrossRef]
- Rajha, H.N.; Abi-Khattar, A.M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of Aqueous Extraction Efficiency and Biological Activities of Polyphenols from Pomegranate Peels Assisted by In-Frared, Ultrasound, Pulsed Electric Fields and High-Voltage Electrical Discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Gumul, D.; Ziobro, R.; Korus, J.; Kruczek, M. Apple Pomace as a Source of Bioactive Polyphenol Compounds in Gluten-Free Breads. Antioxidants 2021, 10, 807. [Google Scholar] [CrossRef]
- Pinto, D.; De La Luz Cádiz-Gurrea, M.; Sut, S.; Ferreira, A.S.; Leyva-Jimenez, F.J.; Dall’acqua, S.; Segura-Carretero, A.; Delerue-Matos, C.; Rodrigues, F. Valorisation of Underexploited Castanea sativa Shells Bioactive Compounds Recovered by Supercritical Fluid Extraction with CO2: A Response Surface Methodology Approach. J. CO2 Util. 2020, 40, 101194. [Google Scholar] [CrossRef]
- Deng, Y.; Ju, T.; Xi, J. Circulating Polyphenols Extraction System with High-Voltage Electrical Discharge: Design and Performance Evaluation. ACS Sustain. Chem. Eng. 2018, 6, 15402–15410. [Google Scholar] [CrossRef]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-Pressure Homogenization Treatment to Recover Bioactive Compounds from Tomato Peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Kehili, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Supercritical CO2 Extraction and Antioxidant Activity of Lycopene and β-Carotene-Enriched Oleoresin from Tomato (Lycopersicum esculentum L.) Peels By-Product of a Tunisian Industry. Food Bioprod. Process. 2017, 102, 340–349. [Google Scholar] [CrossRef]
- Rabelo, R.S.; MacHado, M.T.C.; Martínez, J.; Hubinger, M.D. Ultrasound Assisted Extraction and Nanofiltration of Phenolic Compounds from Artichoke Solid Wastes. J. Food Eng. 2016, 178, 170–180. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L. Effect of Ultrasounds and High Pressure Homogenization on the Extraction of Antioxidant Polyphenols from Lettuce Waste. Innov. Food Sci. Emerg. Technol. 2018. [Google Scholar] [CrossRef]
- Šeregelj, V.; Pezo, L.; Šovljanski, O.; Lević, S.; Nedović, V.; Markov, S.; Tomić, A.; Čanadanović-Brunet, J.; Vulić, J.; Šaponjac, V.T.; et al. New Concept of Fortified Yogurt Formulation with Encapsulated Carrot Waste Extract. LWT 2021, 138, 110732. [Google Scholar] [CrossRef]
- Li, B.; Akram, M.; Al-Zuhair, S.; Elnajjar, E.; Munir, M.T. Subcritical Water Extraction of Phenolics, Antioxidants and Dietary Fibres from Waste Date Pits. J. Environ. Chem. Eng. 2020, 8, 104490. [Google Scholar] [CrossRef]
- Frontuto, D.; Carullo, D.; Harrison, S.M.; Brunton, N.P.; Ferrari, G.; Lyng, J.G.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Polyphenols from Potato Peels Using Response Surface Methodology. Food Bioprocess Technol. 2019, 12, 1708–1720. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mahoonak, A.S.; Mohammadi, A. Application of Gum Arabic and Maltodextrin for Encapsulation of Eggplant Peel Extract as a Natural Antioxidant and Color Source. Int. J. Biol. Macromol. 2019, 140, 59–68. [Google Scholar] [CrossRef]
- Umaña, M.; Turchiuli, C.; Rosselló, C.; Simal, S. Addition of a Mushroom By-Product in Oil-in-Water Emulsions for the Microencapsulation of Sunflower Oil by Spray Drying. Food Chem. 2021, 343, 128429. [Google Scholar] [CrossRef]
- Arrahman, A.; Sigalingging, E.; Trinovita, E.; Saputri, F.C.; Mun’Im, A. Utilization of [Bmim]BF4-MAE on Enhancement of γ-Oryzanol Extraction from Rice Bran and Its Tyrosinase Inhibitory Activity. Braz. J. Pharm. Sci. 2020, 56, 1–11. [Google Scholar] [CrossRef]
- Ahmad, F.; Pasha, I.; Saeed, M.; Asgher, M. Antioxidant Profiling of Native and Modified Cereal Brans. Int. J. Food Sci. Technol. 2019, 54, 1206–1214. [Google Scholar] [CrossRef]
- Sahin, A.W.; Hardiman, K.; Atzler, J.J.; Vogelsang-O’Dwyer, M.; Valdeperez, D.; Münch, S.; Cattaneo, G.; O’Riordan, P.; Arendt, E.K. Rejuvenated Brewer’s Spent Grain: The Impact of Two BSG-Derived Ingredients on Techno-Functional and Nutritional Characteristics of Fibre-Enriched Pasta. Innov. Food Sci. Emerg. Technol. 2021, 68, 102633. [Google Scholar] [CrossRef]
- Merendino, N.; Molinari, R.; Costantini, L.; Mazzucato, A.; Pucci, A.; Bonafaccia, F.; Esti, M.; Ceccantoni, B.; Papeschi, C.; Bonafaccia, G. A New “Functional” Pasta Containing Tartary Buckwheat Sprouts as an Ingredient Improves the Oxidative Status and Normalizes Some Blood Pressure Parameters in Spontaneously Hypertensive Rats. Food Funct. 2014, 5, 1017–1026. [Google Scholar] [CrossRef]
- Larriba, M.; Omar, S.; Navarro, P.; García, J.; Rodríguez, F.; Gonzalez-Miquel, M. Recovery of Tyrosol from Aqueous Streams Using Hydrophobic Ionic Liquids: A First Step towards Developing Sustainable Processes for Olive Mill Wastewater (OMW) Management. RSC Adv. 2016, 6, 18751–18762. [Google Scholar] [CrossRef]
- Simonato, B.; Trevisan, S.; Tolve, R.; Favati, F.; Pasini, G. Pasta Fortification with Olive Pomace: Effects on the Technological Characteristics and Nutritional Properties. LWT 2019, 114, 108368. [Google Scholar] [CrossRef]
- Balli, D.; Cecchi, L.; Innocenti, M.; Bellumori, M.; Mulinacci, N. Food By-Products Valorisation: Grape Pomace and Olive Pomace (Pâté) as Sources of Phenolic Compounds and Fiber for Enrichment of Tagliatelle Pasta. Food Chem. 2021, 355, 129642. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Blouet, C.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Effect of Pulsed Electric Fields and High Voltage Electrical Discharges on Polyphenol and Protein Extraction from Sesame Cake. Innov. Food Sci. Emerg. Technol. 2015, 29, 170–177. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Subcritical Water Extraction of Phenolic and Antioxidant Constituents from Pistachio (Pistacia vera L.) Hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef]
- Carpentieri, S.; Mazza, L.; Nutrizio, M.; Jambrak, A.R.; Ferrari, G.; Pataro, G. Pulsed Electric Fields-and Ultrasound-Assisted Green Extraction of Valuable Compounds from Origanum vulgare L. and Thymus serpyllum L. Int. J. Food Sci. Technol. 2021, 1–9. [Google Scholar]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant Activity of Rosemary (Rosmarinus officinalis L.) Essential Oil and Its Hepatoprotective Potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef] [Green Version]
- Pavlić, B.; Vidović, S.; Vladić, J.; Radosavljević, R.; Cindrić, M.; Zeković, Z. Subcritical Water Extraction of Sage (Salvia officinalis L.) by-Products-Process Optimization by Response Surface Methodology. J. Supercrit. Fluids 2016, 116, 36–45. [Google Scholar] [CrossRef]
- Vladić, J.; Ambrus, R.; Szabó-Révész, P.; Vasić, A.; Cvejin, A.; Pavlić, B.; Vidović, S. Recycling of Filter Tea Industry By-Products: Production of A Millefolium Powder Using Spray Drying Technique. Ind. Crops Prod. 2016, 80, 197–206. [Google Scholar] [CrossRef]
- Gao, J.; You, J.; Kang, J.; Nie, F.; Ji, H.; Liu, S. Recovery of Astaxanthin from Shrimp (Penaeus vannamei) Waste by Ultrasonic-Assisted Extraction Using Ionic Liquid-in-Water Microemulsions. Food Chem. 2020, 325, 126850. [Google Scholar] [CrossRef]
- Tzima, K.; Brunton, N.P.; Lyng, J.G.; Frontuto, D.; Rai, D.K. The Effect of Pulsed Electric Field as a Pre-Treatment Step in Ultrasound Assisted Extraction of Phenolic Compounds from Fresh Rosemary and Thyme By-Products. Innov. Food Sci. Emerg. Technol. 2021, 69, 102644. [Google Scholar] [CrossRef]
- Siemer, C.; Toepfl, S.; Heinz, V. Mass Transport Improvement by PEF-Applications in the Area of Extraction and Distillation. Distill. Adv. from Model. Appl. 2012, 10, 211–232. [Google Scholar]
- Dobrinčić, A.; Repajic, M.; Elez Garofulić, I.; Tuđen, L.; Dragović-Uzelac, V.; Levaj, B. Comparison of Different Extraction Methods for the. Processes 2020, 8, 1008. [Google Scholar] [CrossRef]
- Ahmad, T.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gull, A. Supercritical Fluid Extraction: A Review. J. Biol. Chem. Chron. 2019, 5, 114–122. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional Versus Green Extraction Techniques-A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically Assisted Extraction (UAE) and Microwave Assisted Extraction (MAE) of Functional Compounds from Plant Ma-Terials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Coelho, M.C.; Pereira, R.N.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. The Use of Emergent Technologies to Extract Added Value Compounds from Grape By-Products. Trends Food Sci. Technol. 2020, 106, 182–197. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging Opportunities for the Effective Valorization of Wastes and By-Products Generated During Olive Oil Production Process: Non-Conventional Methods for the Recovery of High-Added Value Compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Niu, D.; Zeng, X.A.; Ren, E.F.; Xu, F.Y.; Li, J.; Wang, M.S.; Wang, R. Review of the Application of Pulsed Electric Fields (PEF) Technology for Food Processing in China. Food Res. Int. 2020, 137, 109715. [Google Scholar] [CrossRef]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New Approaches for the Use of Non-conventional Cell Disruption Technologies to Extract Potential Food Additives and Nutraceuticals from Microalgae. Food Eng. Rev. 2014, 7, 45–62. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A.; Xanthakis, E. The Principles of High Voltage Electric Field and Its Application in Food Processing: A Review. Food Res. Int. 2016, 89, 48–62. [Google Scholar] [CrossRef]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of Alternative Physical Treatments (Ultrasounds, Pulsed Electric Fields, and High-Voltage Electrical Discharges) on Selective Recovery of Bio-compounds from Fermented Grape Pomace. Food Bioprocess Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Khadhraoui, B.; Ummat, V.; Tiwari, B.K.; Fabiano-Tixier, A.S.; Chemat, F. Review of Ultrasound Combinations with Hybrid and Innovative Techniques for Extraction and Processing of Food and Natural Products. Ultrason. Sonochem. 2021, 76, 105625. [Google Scholar] [CrossRef]
- Majid, A.; Phull, A.R.; Khaskheli, A.H.; Abbasi, S.; Sirohi, M.H.; Ahmed, I.; Ujjan, S.H.; Jokhio, I.A.; Ahmed, W. International Journal of Advanced and Applied Sciences Applications and Opportunities of Supercritical Fluid Extraction in Food Processing Technologies: A review. Int. J. Adv. Appl. Sci. 2019, 6, 99–103. [Google Scholar]
- Campone, L.; Celano, R.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Ibañez, E.; Cifuentes, A.; Rastrelli, L. Response Surface Methodology to Optimize Supercritical Carbon Dioxide/Co-Solvent Extraction of Brown Onion Skin By-Product as Source of Nutraceutical Compounds. Food Chem. 2018, 269, 495–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-Assisted Extraction and Ultrasound-Assisted Extraction for Recovering Carotenoids from Gac Peel and Their Effects on Antioxidant Capacity of the Extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of Novel Technologies on Polyphenols During Food Processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing By-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green Alternative Methods for the Extraction of Antioxidant Bioactive Compounds from Winery Wastes and By-Products: A Review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations Guidelines on the Key Information to Be Reported in Studies of Application of PEF Technology in Food and Biotechnological Processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Puértolas, E.; Barba, F.J. Electrotechnologies Applied to Valorization of By-Products from Food Industry: Main Findings, Energy and Economic Cost of Their Industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E. Extraction of Valuable Biocompounds Assisted by High Voltage Electrical Discharges: A Review. Comptes Rendus Chim. 2014, 17, 197–203. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Xi, J. Recent Advances in High Voltage Electric Discharge Extraction of Bioactive Ingredients from Plant Materials. Food Chem. 2019, 277, 246–260. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E.; Reess, T.; De Ferron, A.; Pecastaing, L.; Ruscassié, R.; Lanoisellé, J.L. Scale-Up of High Voltage Electrical Discharges for Polyphenols Extraction from Grape Pomace: Effect of the Dynamic Shock Waves. Innov. Food Sci. Emerg. Technol. 2012, 16, 129–136. [Google Scholar] [CrossRef]
- Donsì, F.; Velikov, K.P. Mechanical Cell Disruption of Mustard Bran Suspensions for Improved Dispersion Properties and Protein Release. Food Funct. 2020, 11, 6273–6284. [Google Scholar] [CrossRef]
- Mesa, J.; Barrera, C.; Betoret, E.; Betoret, N. High Homogenization Pressures to Improve Food. Molecules 2020, 25, 3305. [Google Scholar] [CrossRef]
- Comuzzo, P.; Calligaris, S. Potential Applications of High Pressure Homogenization in Winemaking: A Review. Beverages 2019, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Saini, A.; Panesar, P.S. Beneficiation of Food Processing By-Products through Extraction of Bioactive Compounds Using Neoteric Solvents. LWT 2020, 134, 110263. [Google Scholar] [CrossRef]
- Trinovita, E.; Sigalingging, E.; Saputri, F.C.; Mun’im, A. Optimization of Ionic Liquid 1-Butyl-3-Methylimidazolium Hexafluorophosphate ([Bmim] PF6)-Based Microwave Assisted Ex-Traction Method for Gamma Oryzanol from Rice Bran (Oryza sativa L.). J. Appl. Pharm. Sci. 2017, 7, 8–13. [Google Scholar]
- Liu, Z.; Qiao, L.; Gu, H.; Yang, F.; Yang, L. Development of Brönsted Acidic Ionic Liquid Based Microwave Assisted Method for Simultaneous Extraction of Pectin and Naringin from Pomelo Peels. Sep. Purif. Technol. 2017, 172, 326–337. [Google Scholar] [CrossRef]
- Rachmawati, M.; Ayuningtyas, I.N.; Sutriyo, S.; Mun’im, A. Comparison of Ionic Liquid-Microwave-Assisted Extraction and MAE of Resveratrol from Melinjo (Gnetum gnemon L.) Seeds. J. Appl. Pharm. Sci. 2017, 7, 23–29. [Google Scholar]
- Murador, D.C.; Braga, A.R.C.; Martins, P.L.G.; Mercadante, A.Z.; de Rosso, V.V. Ionic Liquid Associated with Ultrasonic-Assisted Extraction: A New Approach to Obtain Carotenoids from Orange Peel. Food Res. Int. 2019, 126, 108653. [Google Scholar] [CrossRef]
- Wang, T.; Jiao, J.; Gai, Q.Y.; Wang, P.; Guo, N.; Niu, L.L.; Fu, Y.J. Enhanced and Green Extraction Polyphenols and Furanocoumarins from Fig (Ficus carica L.) Leaves Using Deep Eutectic Solvents. J. Pharm. Biomed. Anal. 2017, 145, 339–345. [Google Scholar] [CrossRef]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green Extraction of Polyphenols from Grapefruit Peels Using High Voltage Electrical Discharges, Deep Eutectic Solvents and Aqueous Glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Radojčić Redovniković, I. Enabling Technologies for the Extraction of Grape-Pomace Anthocyanins Using Natural Deep Eutectic Solvents in Up-to-Half-Litre Batches Extraction of Grape-Pomace Anthocyanins Using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef]
- Patsea, M.; Stefou, I.; Grigorakis, S.; Makris, D.P. Screening of Natural Sodium Acetate-Based Low-Transition Temperature Mixtures (LTTMs) for Enhanced Extraction of Antioxidants and Pigments from Red Vinification Solid Wastes. Environ. Process. 2017, 4, 123–135. [Google Scholar] [CrossRef]
- de los Ángeles Fernández, M.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel Approaches Mediated by Tailor-Made Green Solvents for the Extraction of Phenolic Compounds from Agro-Food Industrial By-Products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Pal, C.B.T.; Jadeja, G.C. Microwave-Assisted Deep Eutectic Solvent Extraction of Phenolic Antioxidants from Onion (Allium cepa L.) Peel: A Box–Behnken Design Approach for Optimization. J. Food Sci. Technol. 2019, 56, 4211–4223. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Tzia, C. Extraction of Phenolic Compounds from Olive Pomace by Using Natural Deep Eutectic Solvents and Innovative Extraction Techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Yoo, D.E.; Jeong, K.M.; Han, S.Y.; Kim, E.M.; Jin, Y.; Lee, J. Deep Eutectic Solvent-Based Valorization of Spent Coffee Grounds. Food Chem. 2018, 255, 357–364. [Google Scholar] [CrossRef]
- Mansur, A.R.; Song, N.E.; Jang, H.W.; Lim, T.G.; Yoo, M.; Nam, T.G. Optimizing the Ultrasound-Assisted Deep Eutectic Solvent Extraction of Flavonoids in Common Buckwheat Sprouts. Food Chem. 2019, 293, 438–445. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A. Supercritical Fluid Extraction of Polyphenols from Grape Seed (Vitis vinifera): Study on Process Variables and Kinetics. J. Supercrit. Fluids 2017, 130, 239–245. [Google Scholar] [CrossRef]
- Martinez, G.A.; Rebecchi, S.; Decorti, D.; Domingos, J.M.B.; Natolino, A.; Del Rio, D.; Bertin, L.; Da Porto, C.; Fava, F. Towards Multi-Purpose Biorefinery Platforms for the Valorisation of Red Grape Pomace: Production of Polyphenols, Volatile Fatty Acids, Polyhydroxyalkanoates and Biogas. Green Chem. 2016, 18, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Šojić, B.; Tomović, V.; Kocić-Tanackov, S.; Kovačević, D.B.; Putnik, P.; Mrkonjić, Ž.; Đurović, S.; Jokanović, M.; Ivić, M.; Škaljac, S.; et al. Supercritical Extracts of Wild Thyme (Thymus serpyllum L.) By-Product as Natural Antioxidants in Ground Pork Patties. LWT 2020, 130, 109661. [Google Scholar] [CrossRef]
- Pavlić, B.; Bera, O.; Vidović, S.; Ilić, L.; Zeković, Z. Extraction Kinetics and ANN Simulation of Supercritical Fluid Extraction of Sage Herbal Dust. J. Supercrit. Fluids 2017, 130, 327–336. [Google Scholar] [CrossRef]
- Radzali, S.A.; Masturah, M.; Baharin, B.S.; Rashidi, O.; Rahman, R.A. Optimisation of Supercritical Fluid Extraction of Astaxanthin from Penaeus monodon Waste Using Ethanol-Modified Carbon Dioxide. J. Eng. Sci. Technol. 2016, 11, 722–736. [Google Scholar]
- Santos-Zea, L.; Gutiérrez-Uribe, J.A.; Benedito, J. Effect of Ultrasound Intensification on the Supercritical Fluid Extraction of Phytochemicals from Agave Salmiana bagasse. J. Supercrit. Fluids 2019, 144, 98–107. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Angonese, M.; Gomes, C.; Ferreira, S.R.S. Valorization of Passion Fruit (Passiflora edulis Sp.) By-Products: Sustainable Recovery and Biological Activities. J. Supercrit. Fluids 2016, 111, 55–62. [Google Scholar] [CrossRef]
- Coelho, J.P.; Filipe, R.M.; Paula Robalo, M.; Boyadzhieva, S.; Cholakov, G.S.; Stateva, R.P. Supercritical CO2 Extraction of Spent Coffee Grounds; Influence of Co-Solvents and Characterization of the Extracts. J. Supercrit. Fluids 2020, 161, 104825. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gomez, A.; Serna, J.; Arango, A.; Rubio, S. Supramolecular Solvents for the Valorization of Coffee Wastewater. Environ. Sci. Water Res. Technol. 2020, 6, 757–766. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Supramolecular Solvent Extraction of Bioactives from Coffee Cherry Pulp. J. Food Eng. 2020, 278, 109933. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Sanin, A.; Rubio, S. Valorization of Spent Coffee Grounds by Supramolecular Solvent Extraction. Sep. Purif. Technol. 2019, 228, 115759. [Google Scholar] [CrossRef]
- Pavlić, B.; Naffati, A.; Hojan, T.; Vladić, J.; Zeković, Z.; Vidović, S. Microwave-Assisted Extraction of Wild Apple Fruit Dust-Production of Polyphenol-Rich Extracts from Filter Tea Factory By-Products. J. Food Process Eng. 2017, 40, e12508. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Barreiro, M.F.; Carvalho, A.M.; Oliveira, M.B.P.P.; Curran, T.P.; Ferreira, I.C.F.R. Valorisation of Tomato Wastes for Development of Nutrient-Rich Antioxidant Ingredients: A Sustainable Approach towards the Needs of the Today’s Society. Innov. Food Sci. Emerg. Technol. 2017, 41, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Lasunon, P.; Phonkerd, N.; Tettawong, P.; Sengkhamparn, N. Effect of Microwave-Assisted Extraction on Bioactive Compounds from Industrial Tomato Waste and Its Antioxidant Activity. Food Res. 2021, 5, 468–474. [Google Scholar] [CrossRef]
- Rathnakumar, K.; Anal, A.K.; Lakshmi, K. Optimization of Ultrasonic Assisted Extraction of Bioactive Components from Different Parts of Pineapple Waste. Int. J. Agric. Environ. Biotechnol. 2017, 10, 553. [Google Scholar] [CrossRef]
- Plazzotta, S.; Ibarz, R.; Manzocco, L.; Martín-Belloso, O. Optimizing the Antioxidant Biocompound Recovery from Peach Waste Extraction Assisted by Ultrasounds or Microwaves. Ultrason. Sonochem. 2020, 63, 104954. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cheng, Y.; Chen, P.; Peng, P.; Liu, S.; Li, D.; Ruan, R. Effect of Alkaline and High-Pressure Homogenization on the Extraction of Phenolic Acids from Potato Peels. Innov. Food Sci. Emerg. Technol. 2016, 37, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Gadioli Tarone, A.; Keven Silva, E.; de Freitas Queiroz Barros, H.D.; Baú Betim Cazarin, C.; Roberto Marostica, M., Jr. High-Intensity Ultrasound-Assisted Recovery of Anthocyanins from Jabuticaba By-Products Using Green Solvents: Effects of Ul-Trasound Intensity and Solvent Composition on the Extraction of Phenolic Compounds. Food Res. Int. 2021, 140, 110048. [Google Scholar] [CrossRef] [PubMed]
- Nishad, J.; Saha, S.; Kaur, C. Enzyme-And Ultrasound-Assisted Extractions of Polyphenols from Citrus sinensis (cv. Malta) Peel: A Comparative Study. J. Food Process. Preserv. 2019, 43, e14046. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran Jeganathan, P. Development and Validation of Ultrasound-Assisted Solid-Liquid Extraction of Phenolic Compounds from Waste Spent Coffee Grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Hu, J.; Lu, W.; Lv, M.; Wang, Y.; Ding, R.; Wang, L. Extraction and Purification of Astaxanthin from Shrimp Shells and the Effects of Different Treatments on Its Content. Rev. Bras. Farmacogn. 2019, 29, 24–29. [Google Scholar] [CrossRef]
- Makris, D.P.; Passalidi, V.; Kallithraka, S.; Mourtzinos, I. Optimization of Polyphenol Extraction from Red Grape Pomace Using Aqueous Glycerol/Tartaric Acid Mixtures and Response Surface Methodology. Prep. Biochem. Biotechnol. 2016, 46, 176–182. [Google Scholar] [CrossRef]
- Trasanidou, D.; Apostolakis, A.; Makris, D.P. Development of a Green Process for the Preparation of Antioxidant and Pigment-Enriched Extracts from Winery Solid Wastes Using Response Surface Methodology and Kinetics. Chem. Eng. Commun. 2016, 203, 1317–1325. [Google Scholar] [CrossRef]
- Paleologou, I.; Vasiliou, A.; Grigorakis, S.; Makris, D.P. Optimisation of a Green Ultrasound-Assisted Extraction Process for Potato Peel (Solanum tuberosum) Polyphenols Using Bio-Solvents and Response Surface Methodology. Biomass Convers. Biorefinery 2016, 6, 289–299. [Google Scholar] [CrossRef]
- Katsampa, P.; Valsamedou, E.; Grigorakis, S.; Makris, D.P. A Green Ultrasound-Assisted Extraction Process for the Recovery of Antioxidant Polyphenols and Pigments from Onion Solid Wastes Using Box-Behnken Experimental Design and Kinetics. Ind. Crops Prod. 2015, 77, 535–543. [Google Scholar] [CrossRef]
- Michail, A.; Sigala, P.; Grigorakis, S.; Makris, D.P. Kinetics of Ultrasound-Assisted Polyphenol Extraction from Spent Filter Coffee Using Aqueous Glycerol. Chem. Eng. Commun. 2016, 203, 407–413. [Google Scholar] [CrossRef]
- Rubio, L.; Lamas, J.P.; Lores, M.; Garcia-Jares, C. Matrix Solid-Phase Dispersion Using Limonene as Greener Alternative for Grape Seeds Extraction, Followed by GC-MS Analysis for Varietal Fatty Acid Profiling. Food Anal. Methods 2018, 11, 3235–3242. [Google Scholar] [CrossRef]
- Kehili, M.; Sayadi, S.; Frikha, F.; Zammel, A.; Allouche, N. Optimization of Lycopene Extraction from Tomato Peels Industrial By-Product Using Maceration in Refined Olive Oil. Food Bioprod. Process. 2019, 117, 321–328. [Google Scholar] [CrossRef]
- Varadharajan, V.; Shanmugam, S.; Ramaswamy, A. Model Generation and Process Optimization of Microwave-Assisted Aqueous Extraction of Anthocyanins from Grape Juice Waste. J. Food Process Eng. 2017, 40, e12486. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Extraction Assisted by Pulsed Electric Energy as a Potential Tool for Green and Sustainable Recovery of Nutritionally Valuable Compounds from Mango Peels. Food Chem. 2016, 192, 842–848. [Google Scholar] [CrossRef]
- Ahmad Shiekh, K.; Odunayo Olatunde, O.; Zhang, B.; Huda, N.; Benjakul, S. Pulsed Electric Field Assisted Process for Extraction of Bioactive Compounds from Custard Apple (Annona squamosa) Leaves. Food Chem. 2021, 359, 129976. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound Increases the Aqueous Extraction of Phenolic Compounds with High Antioxidant Activity from Olive Pomace. LWT Food Sci. Technol. 2018, 89, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Xi, J.; He, L.; Yan, L.G. Continuous Extraction of Phenolic Compounds from Pomegranate Peel Using High Voltage Electrical Discharge. Food Chem. 2017, 230, 354–361. [Google Scholar] [CrossRef]
- Anderson, J.L.; Clark, K.D. Ionic Liquids as Tunable Materials in (Bio) Analytical Chemistry. Anal. Bioanal. Chem. 2018, 410, 4565–4566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, J.H.; Tavener, S.J. Alternative Solvents: Shades of Green. Org. Process Res. Dev. 2007, 11, 149–155. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Garrigós, M.C. Il-Based Advanced Techniques for the Extraction of Value-Added Compounds From Natural Sources and Food By-Products. TrAC Trends Anal. Chem. 2019, 119, 115616. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.E.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic Liquid Solutions as Extractive Solvents for Value-Added Compounds from Biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s Solvent Selection Guide-Embedding Sustainability into Solvent Selection Starting at Medicinal Chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Thuy Pham, T.P.; Cho, C.W.; Yun, Y.S. Environmental Fate and Toxicity of Ionic Liquids: A Review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Radošević, K.; Radojčić Redovniković, I.; Halambek, J.; Gaurina Srček, V. A Brief Overview of the Potential Environmental Hazards of Ionic Liquids. Ecotoxicol. Environ. Saf. 2014, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Feng, P. Densities and Viscosities of Ionic Liquid with Organic Solvents. Appl. Sci. 2020, 10, 8342. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Shah, F.U.; Muhammad, N. Efficient Conversion of Lignocellulosic Biomass to Levulinic Acid Using Acidic Ionic Liquids. Carbohydr. Polym. 2018, 181, 208–214. [Google Scholar] [CrossRef]
- Altamash, T.; Amhamed, A.; Aparicio, S.; Atilhan, M. Effect of Hydrogen Bond Donors and Acceptors on CO2 Absorption by Deep Eutectic Solvents. Processes 2020, 8, 1533. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Francisco, M.; Van Den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents. Angew. Chemie Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Radošević, K.; Cvjetko Bubalo, M.; Gaurina Srček, V.; Grgas, D.; Landeka Dragičević, T.; Redovniković, R.I. Evaluation of Toxicity and Biodegradability of Choline Chloride Based Deep Eutectic Solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Mbous, Y.P.; Hayyan, M.; Wong, W.F.; Looi, C.Y.; Hashim, M.A. Unraveling the Cytotoxicity and Metabolic Pathways of Binary Natural Deep Eutectic Solvent Systems. Sci. Rep. 2017, 7, 41257. [Google Scholar] [CrossRef] [PubMed]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which Is the Best Food Emerging Solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The Perspectives of Natural Deep Eutectic Solvents in Agri-Food Sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef]
- Knez, Ž.; Pantić, M.; Cör, D.; Novak, Z.; Knez Hrnčič, M. Are Supercritical Fluids Solvents for the Future? Chem. Eng. Process. Process Intensif. 2019, 141, 107532. [Google Scholar] [CrossRef]
- Cabeza, L.F.; de Gracia, A.; Fernández, A.I.; Farid, M.M. Supercritical CO2 as Heat Transfer Fluid: A Review. Appl. Therm. Eng. 2017, 125, 799–810. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.L.B.; de Aguiar, A.C.; Rostagno, M.A. Extraction of Natural Products Using Supercritical Fluids and Pressurized Liquids Assisted by Ultrasound: Current Status and Trends. Ultrason. Sonochem. 2021, 74, 105584. [Google Scholar] [CrossRef]
- Todd, R.; Baroutian, S. A Techno-Economic Comparison of Subcritical Water, Supercritical CO2 and Organic Solvent Extraction of Bioactives from Grape Marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Vandeponseele, A.; Draye, M.; Piot, C.; Chatel, G. Subcritical Water and Supercritical Carbon Dioxide: Efficient and Selective Eco-Compatible Solvents for Coffee and Coffee By-Products Valorization. Green Chem. 2020, 22, 8544–8571. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized Hot Water Extraction of Bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Castro-Puyana, M.; Marina, M.L.; Plaza, M. Water as Green Extraction Solvent: Principles and Reasons for Its Use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- Giombelli, C.; Iwassa, I.J.; da Silva, C.; Bolanho Barros, B.C. Valorization of peach palm by-product through subcritical water extraction of soluble sugars and phenolic compounds. J. Supercrit. Fluids 2020, 165, 104985. [Google Scholar] [CrossRef]
- Guthrie, F.; Wang, Y.; Neeve, N.; Quek, S.Y.; Mohammadi, K.; Baroutian, S. Recovery of Phenolic Antioxidants from Green Kiwifruit Peel Using Subcritical Water Extraction. Food Bioprod. Process. 2020, 122, 136–144. [Google Scholar] [CrossRef]
- Kheirkhah, H.; Baroutian, S.; Quek, S.Y. Evaluation of Bioactive Compounds Extracted from Hayward Kiwifruit Pomace by Subcritical Water Extraction. Food Bioprod. Process. 2019, 115, 143–153. [Google Scholar] [CrossRef]
- Munir, M.T.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical Water Extraction of Bioactive Compounds from Waste Onion Skin. J. Clean. Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Implementation of Subcritical Water Extraction with Natural Deep Eutectic Solvents for Sustainable Extraction of Phenolic Compounds from Winemaking By-Products. Food Res. Int. 2020, 137, 109728. [Google Scholar] [CrossRef]
- Manna, L.; Bugnone, C.A.; Banchero, M. Valorization of Hazelnut, Coffee and Grape Wastes through Supercritical Fluid Extraction of Triglycerides and Polyphenols. J. Supercrit. Fluids 2015, 104, 204–211. [Google Scholar] [CrossRef]
- Dang, L.H.; Vu, M.T.; Chen, J.; Nguyen, C.K.; Bach, L.G.; Tran, N.Q.; Le, V.T. Effect of Ultrasonication on Self-Assembled Nanostructures Formed by Amphiphilic Positive-Charged Copolymers and Negative-Charged Drug. ACS Omega 2019, 4, 4540–4552. [Google Scholar] [CrossRef]
- Caballo, C.; Sicilia, M.D.; Rubio, S. Supramolecular Solvents for Green Chemistry. Appl. Green Solvents Sep. Process. 2017, 5, 111–137. [Google Scholar]
- Naidu, D.S.; Hlangothi, S.P.; John, M.J. Bio-Based Products from Xylan: A Review. Carbohydr. Polym. 2018, 179, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Han, J. Sustainable Development of Biorefineries: Integrated Assessment Method for Co-Production Pathways. Energy Environ. Sci. 2020, 13, 2233–2242. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anal, A.K. Food Processing By-Products and Their Utilization; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 9781118432938. [Google Scholar]
- Tournour, H.H.; Segundo, M.A.; Magalhães, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of Grape Pomace: Extraction of Bioactive Phenolics with Antioxidant Properties. Ind. Crops Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of Polyphenols from Grape Skins and Defatted Grape Seeds Using Subcritical Water: Experiments and Modeling. Food Bioprod. Process. 2015, 94, 29–38. [Google Scholar] [CrossRef]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. Mathematical Modelling of Anthocyanin Mass Transfer to Predict Extraction in Simulated Red Wine Fermentation Scenarios. Food Res. Int. 2019, 121, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Meullemiestre, A.; Breil, C.; Abert-Vian, M.; Chemat, F. Microwave, Ultrasound, Thermal Treatments, and Bead Milling as Intensification Techniques for Extraction of Lipids from Oleaginous Yarrowia lipolytica Yeast for a Biojetfuel Application. Bioresour. Technol. 2016, 211, 190–199. [Google Scholar] [CrossRef]
- Scurria, A.; Lino, C.; Pitonzo, R.; Pagliaro, M.; Avellone, G.; Ciriminna, R. Vitamin D3 in Fish Oil Extracted with Limonene from Anchovy Leftovers. Chem. Data Collect. 2020, 25, 100311. [Google Scholar] [CrossRef]
- Gali, L.; Bedjou, F.; Velikov, K.P.; Ferrari, G.; Donsì, F. High-Pressure Homogenization-Assisted Extraction of Bioactive Compounds from Ruta chalepensis. J. Food Meas. Charact. 2020, 14, 2800–2809. [Google Scholar] [CrossRef]
- Mustafa, W.; Pataro, G.; Ferrari, G.; Donsì, F. Novel Approaches to Oil Structuring via the Addition of High-Pressure Homogenized Agri-Food Residues and Water Forming Capillary Bridges. J. Food Eng. 2018, 236, 9–18. [Google Scholar] [CrossRef]
- Zhou, T.; McBride, K.; Linke, S.; Song, Z.; Sundmacher, K. Computer-Aided Solvent Selection and Design for Efficient Chemical Processes. Curr. Opin. Chem. Eng. 2020, 27, 35–44. [Google Scholar] [CrossRef]
- Filly, A.; Fabiano-Tixier, A.S.; Fernandez, X.; Chemat, F. Alternative Solvents for Extraction of Food Aromas; Experimental and COSMO-RS Study. LWT Food Sci. Technol. 2015, 61, 33–40. [Google Scholar] [CrossRef]
- Bundeesomchok, K.; Filly, A.; Rakotomanomana, N.; Panichayupakaranant, P.; Chemat, F. Extraction of α-Mangostin from Garcinia mangostana L. Using Alternative Solvents: Computational Predictive and Experimental Studies. LWT Food Sci. Technol. 2016, 65, 297–303. [Google Scholar] [CrossRef]
- Bergez-Lacoste, M.; Thiebaud-Roux, S.; De Caro, P.; Fabre, J.F.; Gerbaud, V.; Mouloungui, Z. From Chemical Platform Molecules to New Biosolvents: Design Engineering as a Substitution Methodology. Biofuels Bioprod. Biorefining 2014, 8, 438–451. [Google Scholar] [CrossRef] [Green Version]
- Weiß, N.; Schmidt, C.H.; Thielemann, G.; Heid, E.; Schröder, C.; Spange, S. The Physical Significance of the Kamlet-Taft: π∗ Parameter of Ionic Liquids. Phys. Chem. Chem. Phys. 2021, 23, 1616–1626. [Google Scholar] [CrossRef]
- Weerachanchai, P.; Chen, Z.; Leong, S.S.J.; Chang, M.W.; Lee, J.M. Hildebrand Solubility Parameters of Ionic Liquids: Effects of Ionic Liquid Type, Temperature and DMA Fraction in Ionic Liquid. Chem. Eng. J. 2012, 213, 356–362. [Google Scholar] [CrossRef]
- del Pilar Sánchez-Camargo, A.; Bueno, M.; Parada-Alfonso, F.; Cifuentes, A.; Ibáñez, E. Hansen Solubility Parameters for Selection of Green Extraction Solvents. TrAC Trends Anal. Chem. 2019, 118, 227–237. [Google Scholar] [CrossRef]
- Pozarska, A.; Da Costa Mathews, C.; Wong, M.; Pencheva, K. Application of COSMO-RS as an Excipient Ranking Tool in Early Formulation Development. Eur. J. Pharm. Sci. 2013, 49, 505–511. [Google Scholar] [CrossRef]
- Touaibia, M.; Fabiano-Tixier, A.-S.; Chemat, F. Chloropinane and Chloromenthene as Novel Solvents for Solubilisation of Natural Substances. Molbank 2021, 2021, M1205. [Google Scholar] [CrossRef]
- Sicaire, A.-G.; Filly, A.; Vian, M.; Fabiano-Tixier, A.-S.; Chemat, F. Cosmo-RS-Assisted Solvent Screening for Green Extraction of Natural Products. Handb. Green Chem. 2018, 12, 117–138. [Google Scholar]
- Klamt, A.; Eckert, F.; Arlt, W. COSMO-RS: An Alternative to Simulation for Calculating Thermodynamic Properties of Liquid Mixtures. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 101–122. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, B.; Winterburn, J.; Gonzalez-Miquel, M. Orange Peel Waste Valorisation through Limonene Extraction Using Bio-Based Solvents. Biochem. Eng. J. 2019, 151, 107298. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J.; Pena-Pereira, F. Environmental Risk-Based Ranking of Solvents Using the Combination of a Multimedia Model and Multi-Criteria Decision Analysis. Green Chem. 2017, 19, 1034–1042. [Google Scholar] [CrossRef] [Green Version]
- Khoo, H.H.; Ee, W.L.; Isoni, V. Bio-Chemicals from Lignocellulose Feedstock: Sustainability, LCA and the Green Conundrum. Green Chem. 2016, 18, 1912–1922. [Google Scholar] [CrossRef]
- Jin, S.; Byrne, F.; McElroy, C.R.; Sherwood, J.; Clark, J.H.; Hunt, A.J. Challenges in the Development of Bio-Based Solvents: A Case Study on methyl (2,2-Dimethyl-1,3-Dioxolan-4-Yl) methyl Carbonate as an Alternative Aprotic Solvent. Faraday Discuss. 2017, 202, 157–173. [Google Scholar] [CrossRef] [Green Version]










| Classification | Advantages | Disadvantages |
|---|---|---|
| Neoteric solvents | Extraction of triglycerides, natural colorants, aromas, polyphenols | |
| Ionic liquids (ILs) [6] | Negligible vapor pressure Thermally stable at temperatures >200 °C Exceptional solubility for organic, inorganic, and organometallic substances | Medium-high viscosity values Some are expensive Toxicity issues not fully addressed |
| Deep Eutectic Solvent (DES) [7,8] | Ease of preparation Excellent solubilization capacity of diverse compounds with poorly water solubility Low cost High biodegradability Adjustable viscosity | High viscosity compared to many conventional organic solvents Toxicity issues not fully addressed |
| Supercritical fluids (SCFs) | Decaffeination of tea and coffee, extraction of lecithin from oil | |
| Supercritical water | Renewability No toxicity issues | High energy requirements in the separation and reuse processes Equipment oxidation issues |
| Supercritical carbon dioxide [9] | Inexpensive No risks associated with the use of organic solvents Odorless, non-toxic, renewable Simple industrial recycling | High pressure required Poor ability to dissolve polar and ionic species High equipment maintenance costs |
| Bio-based solvents | Extraction of pigments, antioxidants | |
| Ethanol [10] | Appreciable solubility of organic compounds in the supercritical state Ease of recovery | Net increase in emissions Flammable and potentially explosive Corrosive in nature |
| Glycerol [11] | Extraction of polyphenols Colorless, odorless, sweet-tasting product and biodegradable Chemical stability during storage High boiling solvent | High operating and investment costs |
| Terpenes [4] | Extraction from fats and oils Ease of recovery and reuse Biodegradability Non-flammability | Low polarity High volatility |
| Supramolecular solvents (SUPRAs) [12] | Extraction of alkaloids, bioactive compounds, removal of pesticides, surfactants, dyes | |
| - | Capability to extract amphiphilic compounds | Extraction of solutes from solid samples not deeply explored |
| Source | Main Antioxidant Compounds | Expected Health-Beneficial Properties | References |
|---|---|---|---|
| Fruit & Vegetables | |||
| Grape pomace (peels, seeds, pulp, stems) | Flavonoids (anthocyanins, monomeric catechin, epicatechin), stilbenes (resveratrol), tannins, gallic acid | Oxidative stress, cancer, and disease risk reduction, cholesterol regulation | [15] |
| Peach residues (peels, seeds, pulp) | Phenolic compounds, carotenoids, vitamin C | Antioxidant, anti-hyperglycemic, anti-aging properties | [16,17] |
| Pineapple residues (peels, stem) | Phenolic compounds, proteolytic enzymes (bromelain), vitamins, carotenoids | Cytotoxic, antidiabetic, antihyperlipidemic, antioxidant properties | [18] |
| Banana peels | Phenols and flavonoids | Inhibition against diverse bacteria and fungi, and some cancer cells, blood sugar, and cholesterol reduction | [19] |
| Mango peels | Carotenoids, flavonoids, phenolic compounds, and vitamins | Reduction of the risk of cancer and coronary heart disease | [20] |
| Pomelo peels | Flavonoids (naringin, quercetin, rutin), vitamins | Antioxidant, anticancer, anti-inflammatory properties, lowering levels of blood cholesterol | [21] |
| Papaya seeds | Tocopherols, carotenoids, flavonoids tannins, fatty acids | Antioxidant properties | [22] |
| Orange peels | Flavonoids (hesperidin, narirutin), carotenoids, xanthophylls | Antioxidant activities, reduction in the incidence of cancer, heart disease, osteoarthritis, ocular disorders | [23] |
| Pomegranate peels | Phenolic compounds (punicalagin) | Antioxidant, anti-inflammatory, hepatoprotective, and antigenotoxic effects | [24] |
| Apple pomace | Phenolic acids (chlorogenic acid), flavonoids (catechins, epicatechins), dihydrochalcone (phloridzin) | Anticancer, anti-inflammatory, antibacterial, and antiviral properties | [25] |
| Chestnut by-products | Vitamin E, phenolic acids, tannins | Antioxidant, anti-inflammatory, and antimicrobial properties | [26] |
| Spent coffee grounds | Phenolic compounds (chlorogenic acid, hydroxyhydroquinone), flavonoids | Antioxidant, anti-inflammatory, anti-microbial, and cholesterol-lowering effects, prevention of degenerative diseases | [27] |
| Tomato pomace | Flavonoids and carotenoids (lycopene) | Reduction of the risk of cardiovascular diseases, atherosclerosis, prostate cancer | [28,29] |
| Artichoke wastes | Phenolic compounds (chlorogenic acid) | Scavenging capacities against reactive oxygen species and reactive nitrogen species, anti-obesity effects | [30] |
| Lettuce waste | Phenolic compounds (chicoric acid; luteolin-7-O-glucuronide) | Antioxidant properties | [31] |
| Carrot pomace | Carotenoids (β-carotene) | Antioxidant, anti-inflammatory properties, improvement of immune response | [32] |
| Onion peels | Flavonoids (quercetin and kaempferol) | Anti-inflammatory and anti-cancer effects | [33] |
| Potato peels | Polyphenols, phenolic acids (caffeic acid, syringic acid) | LDL-lipoprotein oxidation, prevention of platelet aggregation, and red blood cell damage | [34] |
| Eggplant peels | Phenolic compounds, ascorbic acid, anthocyanins (tulipanin, nasunin) | Antioxidant properties | [35] |
| Mushroom stalks | Ergosterol | Antioxidant properties | [36] |
| Cereals | |||
| Rice bran | Tocopherols, tocotrienols, γ-oryzanol, tannins | Antioxidant, antihypertensive, antimicrobial, antidiabetic, anticancer properties, cholesterol-reducing effect | [37] |
| Wheat, barley, millet, sorghum | Phenolic acids, vitamins, minerals | [38] | |
| Brewer’s spent grain | Minerals, vitamins, polyphenols, arabinoxylan, β-glucan | Enhanced glycaemic control, cholesterol-lowering effect, prebiotics effect, immunomodulatory activity, increased minerals absorption | [39] |
| Buckwheat sprouts | Flavonoids (rutin, quercetin), vitamins | Hypocholesterolemic, hypotriglyceridemic, anti-inflammatory properties | [40] |
| Oil crops | |||
| Olive mill wastewater | Tyrosol, hydroxytyrosol | Prevention of Parkinson’s disease, hyperglycemia, cerebral ischemia | [41] |
| Olive pomace | Phenolic compounds, secoiridoids | Antioxidant and anti-inflammatory properties | [42,43] |
| Sesame cake | Polyphenols, lignan glucosides | Prevention of obesity and hyperglycemia, reduction of cholesterol levels | [44] |
| Pistachio hulls | Phenolic acids (gallic acid), gallotannins, flavonoids (quercetin, myricetin glycosides) | Prevention of cardiovascular disease, diabetes, high cholesterol levels | [45] |
| Herbs and spices | |||
| Wild thyme by-product | Flavonoids, phenolic acids, essential oils (thymol) | Antimicrobial, antioxidant, anti-aging, anti-inflammatory, immunomodulatory and anti-cancer, liver protective, gastroprotective activities | [46] |
| Rosemary by-products | Polyphenols (rosmarinic acid, carnosolic acid, carnosol), essential oils | [47] | |
| Sage by-products | [48] | ||
| Tea by-products | Phenolic compounds (chlorogenic acid), flavonoids (apigenin, luteolin), essential oils | [49] | |
| Fish by-products | |||
| Shrimp waste | Carotenoids (astaxanthin) | Antioxidant activity, inhibition of lipid peroxidation | [50] |
| Extraction Method | Advantages | Disadvantages |
|---|---|---|
| SFE | High extraction yields, fast extraction, automated system, no filtration required, possibility to reuse CO2, no use of toxic solvents, possibility to tune the polarity of scCO2, possibility to extract thermolabile compounds at low temperature | High equipment cost, elevated pressure required, risk of volatile compounds losses [54,55] |
| MAE | High extraction yields, small equipment size, easy industrial escalation, low solvent consumption, possibility to develop a solvent-free process, low power consumption, good reproducibility | High equipment cost, non-selective extraction separation, and purification steps required, very poor efficiency for volatile compounds, lack of studies on modeling of the heating process to improve its uniformity [56,57] |
| UAE | Significant savings in maintenance, low equipment cost, low operating temperature, efficient extraction of thermolabile compounds | Separation and purification steps required, lack of uniformity in the distribution of ultrasound energy, potential change in the constitutive molecules, large amount of solvent, difficulty in scaling [58] |
| PEF | Non-destructive, high selectivity, no thermal effect, no need for energy-intensive drying pretreatment, energetically efficient, continuous operability, easy to scale up | Dependence on medium composition (conductivity), high cost of the equipment [59] |
| HPH | High extraction yields, high scalability, ability to overcome high cell wall rigidity, effective in aqueous environments (eliminating the need for energy-intensive drying), one of the most used mechanical methods for large-scale cell disruption | Non-selective extraction, cell debris can bring downstream complications and costs, temperature increase undesirable for heat-sensitive extracts, cooling needed, high energy consumption [60] |
| HVED | High extraction yields, efficient extraction of thermolabile compounds, low solvent consumption, low energy consumption, possibility to extract thermolabile compounds | Batch mode operation, hard to be scaled-up, free radicals would be produced leading to oxidative cell damage, but may also oxidize the target compounds, requires precise control of input energy, less selective than PEF [61,62] |
| Raw Materials | Target Compounds | Emerging Technology | Green or Sustainable Extraction Approach | Main Findings | Reference |
|---|---|---|---|---|---|
| Ionic liquids | |||||
| Olive mill wastewater | Tyrosol | / | [P4441] [Tf2N] and 20% wt sodium chloride T = 70 °C, time = 2 h, L/S = 5 mL/g | Extraction efficiencies higher than 94%, comparable to those of conventional organic solvents | [41] |
| Rice bran | γ-oryzanol | MAE | 0.7 M [Bmim]PF6 solution power = 30%, extraction time = 10 min, L/S = 15 mL/g | IL-MAE method more efficient in extracting 0.27 mg/g of γ-oryzanol than the conventional extraction | [83] |
| 0.7 M [Bmim]BF4 solution power = 30%, extraction time = 10 min, L/S = 15 mL/g | IL-MAE is efficient in extracting γ-oryzanol from rice bran (0.41 mg/g) | [37] | |||
| Pomelo peels | Naringin | MAE | 10 mmol/L [HO3S(CH2)4 mim] HSO4, power = 331 W, time = 15 min, L/S = 26 mL/g | Enhanced extraction yields of 8.38 ± 0.20 mg naringin/g. Reduction of extraction time from 180 min to 15 min | [84] |
| Melinjo (Gnetum gnemon L.) seeds | Resveratrol | MAE | 2.5 mol/L [Bmim] Br; power = 10%; time = 10 min | The antioxidant activity of IL-MAE melinjo seed extract was 82.82% of DPPH inhibition compared to the one of conventional extraction, which inhibits only 5.96% | [85] |
| Shrimp waste | Astaxanthin | UAE | [P4448]Br/(TX-100 + n-butanol)/water Ultrasonic power = 50 W, time = 60 min | ILs enhanced the extraction of astaxanthin due to the stronger electrostatic interactions and hydrogen-bonding compared with organic solvents (extraction yield: 99%) | [50] |
| Orange peels | Carotenoids | UAE | 1-butyl-3-methylimidazolium chloride ([BMIM][Cl]), power = 200 W, f = 20 kHz, 80% amplitude, time = 5 min, L/S = 3 mL/g | Total carotenoid content of 32.08 ± 2.05 μg/g using IL, and 7.88 ± 0.59 μg/g using acetone. IL and carotenoid recovery yields using XAD-7HP resin were 59.5–63.8% and 52.2–58.7% | [86] |
| Deep Eutectic Solvents | |||||
| Fig leaves | Caffeoylmalic acid, psoralic acid-glucoside, rutin, psoralen and bergapten | MAE, UAE | Glycerol, xylitol, and D-Fructose (3:3:3 molar ratio) power = 250 W (MAE) and 700 W (US), time = 10 min (MW) and 60 min (US), T = 40–80 °C | Extraction yields: 6.482 mg/g, 16.34 mg/g, 5.207 mg/g, 15.22 mg/g and 2.475 mg/g, respectively, under optimal extraction conditions (64.46 °C, L/S 17.53 min and 24.43 min using UAE) | [87] |
| Grapefruit peels | Naringin | HVED | Lactic acid:glucose (5:1) HVED as pre-treatment technology (energy 7.27–218 kJ/kg) Solid-liquid extraction T = 50 °C, time = 60 min, L/S = 10 mL/g, | Energy reduction of the HVED pre-treatment by 6 times | [88] |
| Grape pomace | Anthocyanins | Simultaneous UAE and MAE (UMAE) | ChCl:citric acid with 30% water MAE power = 300 W and UAE power = 50 W, time = 10 min, L/S = 33.33 mL/g | The extraction yield of anthocyanins under optimal conditions is 1.77 mg/g DW | [89] |
| Polyphenols | UAE | Sodium acetate:lactic acid molar ratio of 5:1, T = 80 °C, time = 90 min, L/S = 30 mL/g | Total polyphenols yield: 134.54 mg GAE/g DW | [90] | |
| Onion, tomato, pear, and olive industrial by-products | Polyphenols | UAE | Lactic acid:glucose (5:1) with 15% water L/S = 75 mL/g | Simple, non-expensive, eco-friendly, and robust system. The application to different matrices demonstrated the versatility of the proposed method | [91] |
| Onion peels | Polyphenols | MAE | ChCl:urea:water (1:2:4) power = 100 W, time = 15.03 min, L/S = 54.97 mL/g | MAE allowed a recovery of bioactive compounds (80.45 mg GAE/g) 1.5 times higher than conventional extraction with 24-fold reduction in extraction time | [92] |
| Olive pomace | Polyphenols | HAE, MAE, UAE, HHPAE | ChCl:maltose (1:2); ChCl:glycerol (1:2) Homogenate–(HAE), microwave–(MAE), ultrasound–(UAE) or high hydrostatic pressure–(HHPAE) assisted extractions, T = 60 °C, time = 30 min, 12,000 rpm, L/S = 12.5 mL/g | HAE proved to be the best method with extraction efficiency superior to MAE, UAE, and HHPAE | [93] |
| Spent coffee ground | Chlorogenic acids and flavonoids | UAE | 1,6-hexanediol:ChCl molar ratio 7:1 (HC-6) 67.5% w/w, T = 60 °C, time = 10 min, L/S = 26 mL/g | Significantly higher extraction efficiency compared to conventional methods using water or aqueous organic solvents | [94] |
| Buckwheat sprouts | Flavonoids | UAE | 80% CCTG (CC-based DES composed of triethylene glycol and 20 vol% water), T = 56 °C, time = 40 min, power = 700 W, f = 40 kHz | DES coupled with UAE is a valuable alternative for the green extraction of flavonoids from buckwheat spouts | [95] |
| Supercritical fluids | |||||
| Grape seeds | Polyphenols | SFE | T = 40 °C, P = 80 bar, flow rate = 6 kg CO2/h, co-solvent = 20% (w/w) ethanol-water | Extraction yield of total polyphenols: 7.1 g GAE/100 g dry matter | [96] |
| Red grape pomace | Polyphenols, volatile fatty acids, polyhydroxyalkanoates, biogas | SFE | T = 40 °C, P = 80 bar, flow rate = 6 kg CO2/h, co-solvent = 57% (w/w) ethanol-water | Extraction yield of total polyphenols: 2.7 g GAE/100 g dry matter | [97] |
| Wild thyme by-product | Polyphenols | SFE | SFE1 P = 100 bar, T = 40 °C and SFE2 P = 350 bar, T = 50 °C | Promising natural antioxidants and antimicrobial agents in meat processing (0.075 μL/g ground pork patties) | [98] |
| Tomatoes peels and seeds | Carotenoids | SFE | T = 80 °C, P = 400 bar, flow rate = 4 g CO2/min, time = 2 h | Extraction yield: 410.53 mg lycopene/kg, and 31.38 mg β-carotene/kg from peels, 27.84 mg lycopene/kg, and 5.25 mg β-carotene/kg from seeds, on dry weights | [29] |
| Castanea sativa shells | Ellagic acid, epigallocatechin, catechin, caffeic acid derivative | SFE | T = 60 °C, P = 350 bar, CO2, 15% (v/v) ethanol as co-solvent | Extract as promising nutraceutical ingredient and effective scavenger of NO radical and HOCl | [26] |
| By-products from filter-tea factory (sage herbal dust) | Diterpene polyphenols | SFE | T = 40 and 60 °C, P = 100–300 bar, flow rate = 0.4 CO2 kg/h, time = 5 h | SFE process at 283 bar and 60 °C provided the highest extraction yield of the investigated compounds | [99] |
| Penaeus monodon waste | Astaxanthin | SFE | 15% (v/v) ethanol as co-solvent, T = 56.88 °C, P = 215.68 bar, time = 120 min, flow rate = 1.89 mL CO2/min | Recovery yield of 58.50 ± 2.62 µg/g astaxanthin and 12.20 ± 4.16 µg/g free astaxanthin | [100] |
| Agave bagasse | Antioxidants and saponins | SFE + UAE | 10% (v/v) ethanol as co-solvent, T = 60 °C, P = 300 bar | Antioxidant capacity from 12.18 ± 1.01 to 20.91 ± 1.66 μmol TE/g when using UAE | [101] |
| Passion fruit seeds and seed cake | Oil and extract with promising antioxidant and antimicrobial activities | SFE | T = 40–50 °C, P = 150, 250 and 300 bar, time = 2.5–3 h, flow rate = 0.5 kg CO2/h | The best yields obtained by SFE at 250 bar/40 °C for the seed (27 ± 1%) and by cold maceration (with EtOH–H2O (1/1, v/v) for the seed cake (6 ± 1%) | [102] |
| Spent coffee grounds | Oil fraction, antioxidants | SFE | T = 39.85 °C and 59.85 °C, P = up to 50.0 MPa, flow rate = 1.9 × 10−3 kg CO2/min, co-solvents: isopropanol, ethanol and ethyl lactate | Co-solvents decreased the extraction time to half of that with pure CO2 and increased the antioxidant capacity by up to 12.5 times | [103] |
| Supramolecular solvents | |||||
| Coffee wastewater | Caffeine | / | Amphiphile: 1-hexanol or decanoic acid = 2.9–17.1% v/v, ethanol = 3.8–46.2% v/v, time = 20 min | Caffeine yield: 54–65 mg/L of wastewater. Good antioxidant activity (up to 53%) | [104] |
| Coffee cherry pulp | Phenolic and alkaloid compounds | / | Amphiphile: decanoic or octanoic acid, L/S = 4:1 v/w, time = 5 min | Extraction yield: 3.6 ± 0.3 mg caffeine g−1, 0.9 ± 0.1 mg protocatechuic acid g−1 | [105] |
| Spent Coffee grounds | Caffeine, 5-CGA, and total phenolic compounds | / | Amphiphile: 1-Hexanol, decanoic acid, 24% v/v 1-hexanol, 30% v/v ethanol and 46% v/v water, time = 1 min | Extraction yield: 3.32 mg caffeine g−1; 4.3 mg chlorogenic acid g−1; 60.1 mg 5-GAE g−1 (Total phenolic compounds) | [106] |
| Bio-based solvents | |||||
| Ethanol | |||||
| Apple dust by-product from filter tea factory | Polyphenols and antioxidants | MAE | Ethanol = 40–80% v/v time = 15–35 min, power = 400–800 W | Best extraction conditions: 15.2 min, ethanol concentration of 40% and microwave irradiation of 400 W | [107] |
| Tomato pericarps | Nutrient-rich antioxidant ingredients | MAE | Ethanol = 0–100% v/v, time = 0–20 min, T = 60–180 °C, L/S = 22 mL/g, power = 200 W | Extraction yield of 75.5% and ingredients with high levels of sugars, proteins, phenolics, and flavonoids | [108] |
| Tomato waste | Trans-lycopene, beta-carotene phenolics and flavonoids | MAE | Ethanol = 95% v/v, L/S = 20 mL/g, power = 180, 300, 450 W, time = 30, 60 and 90 s | 300 W for 60 s was the best condition that gave the high quality for bioactive compounds | [109] |
| Pineapple waste | Polyphenols, antioxidants | UAE | Ethanol = 0, 20 and 40% v/v, L/S = 10 mL/g, US mode = 0.5, time = 10, 20, 30 min, power = 200 W | UAE and ethanol as a solvent effective method for the extraction of bioactive compound | [110] |
| Artichoke wastes | Phenolic compounds | UAE | Ethanol = 50% v/v, L/S = 10 mL/g, time = 10 min, power = 240 W | UAE favoured the extraction of phenolic compounds, but power > 240 W had no influence on process efficiency | [30] |
| Peach waste | Total phenolic content, total flavonoid, anthocyanins | UAE, MAE | Ethanol = 70% v/v, MAE power = 540 W, UAE power = 23%, MAE time = 50 s, UAE time = 120 s | Comparable extraction efficiency. However, vitamin C was successfully extracted only by MAE, due to oxidative degradation during UAE | [111] |
| Peach waste | Total phenolic content, total flavonoid, anthocyanins | PEF | Ethanol = 70% v/v, W = 0.0014 kJ/kg, treatment time = 16 μs | PEF led to a reduction of extraction times (16 μs), compared to thermal extraction (40 min), reaching the same yields | [16] |
| Pomelo peels | Naringin | PEF | E = 4 kV/cm, pulses = 30, L/S = 90 mL/g, solvent = ethanol 40% v/v, T = 40 °C | PEF improved the extraction yields of naringin by 20% compared with the untreated sample | [21] |
| Lettuce waste | Polyphenols | HPH, UAE | Ethanol = 50–75% v/v, HPH: P = 50 MPa, US: P = 400 W, f = 24 kHz, time = 120 s, L/S = 50 mL/g, | HPH led to a reduction in phenolic yields compared to UAE, possibly due to the 40% activation of polyphenol oxidase | [31] |
| Potato peels | Phenolic acids | HPH | L/S = 25 mL/g in ethanol and NaOH (0–0.4 mol/L), T = 40 °C, P = 158.58 MPa, n 2 passes | The combination of NaOH and HPH improved the extraction yield of total phenolic acid. The highest contribution is associated with HPH | [112] |
| Fresh rosemary and thyme by-products | Phenolics | PEF pre-treatment, then, UAE | PEF: n = 167, pulse width = 30 µ, 0.1% aqueous NaCl, L/S = 1.4 v/w for rosemary, and 1.5 v/w for thyme, E = 1.1 ± 0.2 kV cm−1, W = 0.36 and 0.46 kJ kg−1 for rosemary and thyme US: T = 40 °C, P = 200 W, Ethanol = 55.19% v/v, L/S = 20 mL/g, time = 12.48 min | PEF pre-treatment enhanced (p < 0.05) the recovery of phenolics and antioxidant activity compared to US individually | [51] |
| Jabuticaba peels | Anthocyanins, pectin | UAE | UAE intensity = 3.7 W/cm2, Ethanol = 50% v/v, L/S = 25 mL/g | The synergy between UAE and the solvent strongly influenced the extraction efficiency of anthocyanins | [113] |
| Citrus peels | Polyphenols (TPC), flavonoids (TFC) | UAE | 70.89% amplitude, L/S = 40 mL/g, time = 35 min | TPC and TFC yield of 1590 ± 0.92 mg GAE/100 g and 104.99 ± 0.35 mg QE/100 g, respectively | [114] |
| Mushroom stalks | Ergosterol and antioxidant components | UAE | Ethanol = 70 and 96% v/v, power density = 182 ± 7 W/L, 321 ± 14 W/L, L/S = 5 mL/g | Extraction yield increases up to 2 times in ergosterol, 46% in phenolic compounds, and 25% in antioxidant activity | [36] |
| Spent coffee grounds | Chlorogenic acid (CGA), protocatechuic acid (PCA) | UAE | Power = 244 W, T = 40 °C, time = 40 min, L/S = 17 mL/g | Extraction yield: 1.34 ± 0.37 mg/g of CGA and 0.51 ± 0.03 mg/g of PCA | [115] |
| Shrimp shells | Astaxanthin | UAE | L/S = 7 mL/g, time = 20 min, T = 50 °C, f = 40 kHz | Extraction yield is 43.7 g/g. The purity of the obtained astaxanthin was 85.1% using silica gel column chromatography | [116] |
| Spent coffee grounds | Polyphenols | HVED | Ethanol = 24% v/v; peak voltage = 11 kV; flow rate = 12 L/h; L/S = 15 mL/g; time = 20 min | Extraction yields are higher by 20.03% than solvent extraction. Reduced extraction time (by 87%) and energy consumption (by 65%) | [27] |
| Glycerol | |||||
| Red grape pomace | Polyphenols, flavonoids | / | T = 23 °C, time = 180 min, S/L = 50 mL/g | Aqueous glycerol (20%, w/v) is suitable for retrieving polyphenols, flavonoids, and pigments from grape pomace | [117] |
| UAE | Power = 140 W, f = 37 kHz, time = 60 min, T = 45 °C, Glycerol = 90% (w/v), L/S = 90 mL/g | Aqueous glycerol in combination with UAE can efficiently extract polyphenols and pigments | [118] | ||
| Potato peels | Polyphenolic antioxidants | UAE | Power = 140 W, f = 37 kHz, time = 90 min, Water/glycerol: glycerol = 83% (w/v), L/S = 81 mL/g, T = 80 °C Water/ethanol: ethanol = 59% (v/v), L/S = 84 mL/g, T = 77 °C | Extraction yield in total polyphenols: 8.71 and 9.11 mg caffeic acid/g dry weight, for water/glycerol and water/ethanol mixtures, respectively | [119] |
| Onion wastes | Polyphenols, flavonoids | UAE | Glycerol = 90% (w/v), T = 50 °C; time = 60 min; L/S = 90 mL/g | Aqueous glycerol UAE efficiently extracted polyphenols from onion wastes (yield: 90.07 mg GAE/g) | [120] |
| Spent filter coffee | Polyphenols | UAE | Glycerol 3.6% (w/v), T = 45 °C; time = 175 min; L/S = 50 mL/g | Aqueous glycerol efficiently provided a higher total polyphenol yield (7.4%) compared to water | [121] |
| Limonene | |||||
| Grape seeds | Fatty acids | / | 32% limonene, 35% ethyl acetate, 33% MTBE | The use of limonene allowed obtaining similar yields to longer extraction procedures using organic solvents | [122] |
| Olive oil | |||||
| Tomato peels | Lycopene | / | T = 80 °C, time = 45 min, agitation speeds = 400 rpm, L/S = 0.4% (v/w) | Extraction yield: 99.3% of the initial lycopene content. Olive oil represents a green solution that prevents lycopene from lipid oxidation | [123] |
| Water | |||||
| Banana peel | Phenolics | MAE | pH = 1, time = 6 min, power = 960 W, L/S = 50 mL/g | Water effectively recovered phenolic compounds (50.55 mg/g dried peel) from banana peel using MAE | [19] |
| Grape juice waste | Anthocyanins | MAE | Time = 1–5 min, power = 100–600 W, L/S = 10–50 mL/g | Extraction anthocyanin yield: 1.3215 mg/g of grape waste at the power of 435 W, time of 2.31 min, L/S = 19.22 mL/g | [124] |
| Mango peels | Polyphenols, proteins, carbohydrates | PEF, HVED | Electric field strength (PEF) = 13.3 kV/cm, (HVED) = 40 kV/cm, n = 2000, W = 1000 kJ/kg distance between pulses = 2 s, T = 20 °C, L/S = 10 (w/w) | HVED is more effective than PEF, however, PEF is more selective | [125] |
| Fermented grape pomace | Total phenolic compounds, anthocyanins | UAE, PEF, HVED | US: power = 400 W f = 24 kHz PEF: E = 13.3 kV/cm, W = 0–564 kJ/kg, HVED: W = 0–218 kJ/kg, L/S = 10 mL/g | HVED led to the highest phenolic compound’s recovery with lower energy requirement than PEF and US | [62] |
| Tomato peels | Polyphenols, proteins | HPH | P = 100 MPa, n =10 passes, L/S = 10 mL/g | Increase in proteins (+70.5%), polyphenols (+32.2%), antioxidant activity (+23.3%) | [28] |
| Potato peels | Phenolic compounds | PEF | Pre-treatment: E = 1 kV/cm, W = 5 kJ/kg, treatment time = 6 ms, L/S = 1 mL water/g S/L extraction: Ethanol = 52%, time = 230 min, T = 50 °C | PEF reduced time, temperature, and solvent, improved the extraction yield (10%) and antioxidant activity (9%) than the untreated sample | [34] |
| Custard apple leaves | Phenolic compounds | PEF | Pre-treatment: E = 2, 4 or 6 kV/cm, W = 45, 94 or 142 kJ/kg, treatment time = 2.5–5 min, L/S = 2.5 mL/g S/L extraction: Ethanol = 70, L/S = 15:1 (v/w) | PEF improved the extraction yields (+5.2%) and the antioxidant activity than the untreated sample | [126] |
| Olive pomace | Phenolic compounds | UAE | Power = 250 W, time = 75 min, T = 30 °C, L/S = 50 mL/g | UAE increased the extraction yield of phenolic compounds of 30% compared to the the control | [127] |
| Sesame cake | Polyphenols, proteins | PEF, HVED | Pre-treatment: E = 13.3 kV/cm, W = 83 kJ/kg, treatment time = 1–7 ms, holding time = 4–28 min, T = 20–60 °C, L/S = 10 mL/g S/L extraction: Ethanol = 10%, L/S = 20 mL/g, time = 1 h | PEF and HVED accelerated the diffusion kinetics, making the impact of temperature smaller | [44] |
| Pomegranate peel | Phenolic compounds | HVED | T = 25 °C; peak voltage = 9 kV; flow rate = 12 mL/min; L/S = 35 mL/g; electrodes distance = 3.1 mm; time = 30 min | Extraction yield: 196.7 ± 6.4 mg/g. HVED is more efficient in extracting phenolic compounds than the warm water maceration | [128] |
| Selectivity Criteria | Predicted Properties | Advantages | Limitations | References |
|---|---|---|---|---|
| Kauri-butanol index | Relative solvency power of a solvent, based on the maximum amount of solvent added to a solution of Kauri gum in n-butanol without causing cloudiness | Simple model | Provides a scaleless index. Not suitable for oils and fats. Sometimes inconsistent with theoretical results. Conducting the test under conditions other than 25 °C, 1 atm yields different results | [177] |
| Kamlet-Taft scale | Hydrogen bond donation ability (α), hydrogen bond acceptor ability (β), dipolarity-polarizability (π*) | Simple scale-based model. Widely used multiparameter scale | Sometimes inconsistent results | [177,178] |
| Hildebrand solubility parameters | Interaction degree between chemicals, relative solvency behavior | Simple predictive theory Good indication of solubility, especially for nonpolar or slightly polar systems without hydrogen bonding | Not suitable for polar systems | [179] |
| HSPs | Total cohesive energy density as the result of the combination of three intermolecular interactions | Powerful indicator of predicted solubility | Physicochemical properties of some “green” solvents are insufficiently investigated. More complicated three-dimensional solubility parameters | [180] |
| COSMO-RS | Molecular polarity distribution accordingly integrated to calculate the chemical potential of the surface (σ-potential) | Very accurate method, very robust and valuable tool. Applied in a wide range of industrial applications | The quantum chemistry calculation step requires expertise as well as a significant computational time | [181] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. https://doi.org/10.3390/antiox10091417
Carpentieri S, Soltanipour F, Ferrari G, Pataro G, Donsì F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants. 2021; 10(9):1417. https://doi.org/10.3390/antiox10091417
Chicago/Turabian StyleCarpentieri, Serena, Farid Soltanipour, Giovanna Ferrari, Gianpiero Pataro, and Francesco Donsì. 2021. "Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry" Antioxidants 10, no. 9: 1417. https://doi.org/10.3390/antiox10091417
APA StyleCarpentieri, S., Soltanipour, F., Ferrari, G., Pataro, G., & Donsì, F. (2021). Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants, 10(9), 1417. https://doi.org/10.3390/antiox10091417