Lipid-Encapsuled Grape Tannins Prevent Oxidative-Stress-Induced Neuronal Cell Death, Intracellular ROS Accumulation and Inflammation
Abstract
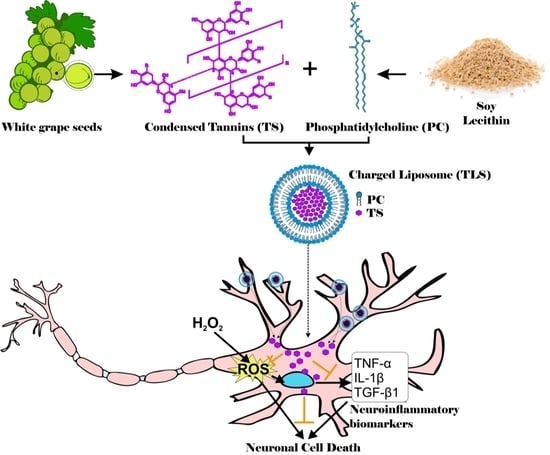
:1. Introduction
2. Materials and Methods
2.1. Liposome Preparation and Tannins Encapsulation
2.2. Mean Particle Size (MPS), Polydispersity Index (PDI), and z-Potential (ξ)
2.3. Proximal Composition
2.4. Quantification of Total Phenols
2.5. HPLC-UV
2.6. Trolox Equivalent Antioxidant Capacity Assay
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. Intracellular ROS Assay
2.10. RNA Isolation, cDNA Synthesis and RT-qPCR Analysis of Neuroinflammatory Biomarkers
2.11. Statistical Analysis
3. Results
3.1. Lipid-Encapsulated Tannins Characterization
3.2. Tannins and Charged Liposomes Prevent Hydrogen Peroxide-Induced Cell Death
3.3. Tannins and Charged Liposomes Prevented Intracellular ROS Production after Hydrogen Peroxide Trearment
3.4. Lipid-Encapsulated Grape Tannins Protects Neurons against ROS-Induced Neuroinflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox. Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [CrossRef]
- Díaz, H.S.; Toledo, C.; Andrade, D.C.; Marcus, N.J.; Del Rio, R. Neuroinflammation in heart failure: New insights for an old disease. J. Physiol. 2020, 598, 33–59. [Google Scholar] [CrossRef]
- Itoh, K.; Nakamura, K.; Iijima, M.; Sesaki, H. Mitochondrial dynamics in neurodegeneration. Trends Cell Biol. 2013, 23, 64–71. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Moylan, J.S.; Reid, M.B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 2007, 35, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.K.; Hilsabeck, T.; Rea, S.L. The role of mitochondrial dysfunction in age-related diseases. Biochim. Biophys. Acta 2015, 1847, 1387–1400. [Google Scholar] [CrossRef]
- Lehnardt, S.; Massillon, L.; Follett, P.; Jensen, F.E.; Ratan, R.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 8514–8519. [Google Scholar] [CrossRef]
- Zhu, Y.; Carvey, P.M.; Ling, Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006, 1090, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox. Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.M.; Hutchinson, M.; Watkins, L.R.; Yin, H. Toll-like receptor 4 in CNS pathologies. J. Neurochem. 2010, 114, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Abou-Sleiman, P.M.; Muqit, M.M.K.; Wood, N.W. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat. Rev. Neurosci. 2006, 7, 207–219. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen Res. 2018, 13, 2055–2059. [Google Scholar] [CrossRef]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; D’Errico, S.; Ciccone, R.; De Feo, V.; Secondo, A.; Pannaccione, A. Exploring the Therapeutic Potential of Phytochemicals in Alzheimer’s Disease: Focus on Polyphenols and Monoterpenes. Front. Pharmacol. 2022, 13, 876614. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 5137431. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [Green Version]
- Hirschberg, S.; Gisevius, B.; Duscha, A.; Haghikia, A. Implications of Diet and The Gut Microbiome in Neuroinflammatory and Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3109. [Google Scholar] [CrossRef] [PubMed]
- Roumes, H.; Sanchez, S.; Benkhaled, I.; Fernandez, V.; Goudeneche, P.; Perrin, F.; Pellerin, L.; Guillard, J.; Bouzier-Sore, A.K. Neuroprotective Effect of Eco-Sustainably Extracted Grape Polyphenols in Neonatal Hypoxia-Ischemia. Nutrients 2022, 14, 773. [Google Scholar] [CrossRef] [PubMed]
- El Gaamouch, F.; Liu, K.; Lin, H.Y.; Wu, C.; Wang, J. Development of grape polyphenols as multi-targeting strategies for Alzheimer’s disease. Neurochem. Int. 2021, 147, 105046. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Gawel, R. Red wine astringency: A review. Aust. J. Grape Wine Res. 1998, 4, 74–95. [Google Scholar] [CrossRef]
- Somers, T.J.P. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Ullah, R.; Ali, G.; Baseer, A.; Irum Khan, S.; Akram, M.; Khan, S.; Ahmad, N.; Farooq, U.; Kanwal Nawaz, N.; Shaheen, S.; et al. Tannic acid inhibits lipopolysaccharide-induced cognitive impairment in adult mice by targeting multiple pathological features. Int. Immunopharmacol. 2022, 110, 108970. [Google Scholar] [CrossRef]
- Mori, T.; Rezai-Zadeh, K.; Koyama, N.; Arendash, G.W.; Yamaguchi, H.; Kakuda, N.; Horikoshi-Sakuraba, Y.; Tan, J.; Town, T. Tannic acid is a natural β-secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice. J. Biol. Chem. 2012, 287, 6912–6927. [Google Scholar] [CrossRef] [Green Version]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.; Miroshnyk, I.; Habib, M.J.; Brausch, J.F.; Hussain, M.D. Enhanced Dissolution and Oral Bioavailability of Piroxicam Formulations: Modulating Effect of Phospholipids. Pharmaceutics 2010, 2, 339–350. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, X.; Lu, Y.; Shi, S.; Yang, D.; Yao, T. New Strategy for Reducing Tau Aggregation Cytologically by a Hairpinlike Molecular Inhibitor, Tannic Acid Encapsulated in Liposome. ACS Chem. Neurosci. 2020, 11, 3623–3634. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; De Pasquale, D.; Battaglini, M.; di Leo, N.; Degl’Innocenti, A.; Belenli Gümüş, M.; Drago, F.; Ciofani, G. Tannic Acid–Iron Complex-Based Nanoparticles as a Novel Tool against Oxidative Stress. ACS Appl. Mater. Interfaces 2022, 14, 15927–15941. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, J.K.; McMillian, M.; Chikaraishi, D.M. Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J. Neurosci. 1997, 17, 1217–1225. [Google Scholar] [CrossRef]
- Alemán, A.; Mastrogiacomo, I.; López-Caballero, M.E.; Ferrari, B.; Montero, M.P.; Gómez-Guillén, M.C. A Novel Functional Wrapping Design by Complexation of ε-Polylysine with Liposomes Entrapping Bioactive Peptides. Food Bioproc. Tech. 2016, 9, 1113–1124. [Google Scholar] [CrossRef]
- Taladrid, D.; Marín, D.; Alemán, A.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Effect of chemical composition and sonication procedure on properties of food-grade soy lecithin liposomes with added glycerol. Food Res. Int. 2017, 100, 541–550. [Google Scholar] [CrossRef]
- Lopez-Polo, J.; Silva-Weiss, A.; Giménez, B.; Cantero-López, P.; Vega, R.; Osorio, F.A. Effect of lyophilization on the physicochemical and rheological properties of food grade liposomes that encapsulate rutin. Food Res. Int. 2020, 130, 108967. [Google Scholar] [CrossRef]
- Cano, A.; Andres, M.; Chiralt, A.; González-Martinez, C. Use of tannins to enhance the functional properties of protein based films. Food Hydrocoll. 2020, 100, 105443. [Google Scholar] [CrossRef]
- Bianchi, S.; Kroslakova, I.; Mayer, I. Determination of Molecular Structures of Condensed Tannins from Plant Tissues Using HPLC-UV Combined with Thiolysis and MALDI-TOF Mass Spectrometry. Bio-Protoc 2016, 6, e1975. [Google Scholar] [CrossRef]
- Compaoré, M.; Meda, R.N.-T.; Bakasso, S.; Vlase, L.; Kiendrebeogo, M. Antioxidative, anti-inflammatory potentials and phytochemical profile of Commiphora africana (A. Rich.) Engl. (Burseraceae) and Loeseneriella africana (Willd.) (Celastraceae) stem leaves extracts. Asian Pac. J. Trop. Biomed. 2016, 6, 665–670. [Google Scholar] [CrossRef]
- Díaz, H.S.; Andrade, D.C.; Toledo, C.; Schwarz, K.G.; Pereyra, K.V.; Díaz-Jara, E.; Marcus, N.J.; Rio, R.D. Inhibition of Brainstem Endoplasmic Reticulum Stress Rescues Cardiorespiratory Dysfunction in High Output Heart Failure. Hypertension 2021, 77, 718–728. [Google Scholar] [CrossRef]
- Díaz-Jara, E.; Díaz, H.S.; Rios-Gallardo, A.; Ortolani, D.; Andrade, D.C.; Toledo, C.; Pereyra, K.V.; Schwarz, K.; Ramirez, G.; Ortiz, F.C.; et al. Exercise training reduces brainstem oxidative stress and restores normal breathing function in heart failure. Free Radic. Biol. Med. 2021, 172, 470–481. [Google Scholar] [CrossRef]
- Czaplicka, M.; Parypa, K.; Szewczuk, A.; Gudarowska, E.; Rowińska, M.; Zubaidi, M.A.; Nawirska-Olszańska, A. Assessment of Selected Parameters for Determining the Internal Quality of White Grape Cultivars Grown in Cold Climates. Appl. Sci. 2022, 12, 5534. [Google Scholar] [CrossRef]
- Lin, C.C.; Lee, I.T.; Wu, W.L.; Lin, W.N.; Yang, C.M. Adenosine triphosphate regulates NADPH oxidase activity leading to hydrogen peroxide production and COX-2/PGE2 expression in A549 cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L401–L412. [Google Scholar] [CrossRef]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Carson, M.J.; Thrash, J.C.; Walter, B. The cellular response in neuroinflammation: The role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin. Neurosci. Res. 2006, 6, 237–245. [Google Scholar] [CrossRef]
- Lawrimore, C.J.; Crews, F.T. Ethanol, TLR3, and TLR4 Agonists Have Unique Innate Immune Responses in Neuron-Like SH-SY5Y and Microglia-Like BV2. Alcohol. Clin. Exp. Res. 2017, 41, 939–954. [Google Scholar] [CrossRef] [Green Version]
- Pandur, E.; Varga, E.; Tamási, K.; Pap, R.; Nagy, J.; Sipos, K. Effect of Inflammatory Mediators Lipopolysaccharide and Lipoteichoic Acid on Iron Metabolism of Differentiated SH-SY5Y Cells Alters in the Presence of BV-2 Microglia. Int. J. Mol. Sci. 2018, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Monnet-Tschudi, F.; Defaux, A.; Braissant, O.; Cagnon, L.; Zurich, M.-G. Methods to assess neuroinflammation. Curr. Protoc. Toxicol. 2011, 50, 12.19.1–12.19.20. [Google Scholar] [CrossRef] [PubMed]



| Analysis | TS | TLS |
|---|---|---|
| Particle size Mean particle size (MPS) Polydispersity index (PDI) Z-potential (ζ) Proximal Analysis | 742.7 ± 5.30 nm 0.67 ± 0.03 −13.50 ± 1.70 mV Content (g/100 g) | 309.9 ± 5.90 nm 0.41 ± 0.02 −21.40 ± 1.70 mV Content (g/100 g) |
| Moisture Ash | 95.06 0.57 | 96.64 0.57 |
| Protein | 0.08 | 0.08 |
| Lipids | 0.48 | 0.18 |
| N.N.E | 2.64 | 2.53 |
| Kcal/100 g | 11.39 | 12.04 |
| GAE (µg/mL) | GAE (µg/mL) | |
| Total polyphenols | 78.34 ± 0.12 * | 30.23 ± 0.14 |
| TEAC (µmol TE/mL) | TEAC (µmol TE/mL) | |
| Antioxidant activity (ABTS) | 2017.51 ± 238.57 * | 9.10 ± 6.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, H.S.; Ríos-Gallardo, A.; Ortolani, D.; Díaz-Jara, E.; Flores, M.J.; Vera, I.; Monasterio, A.; Ortiz, F.C.; Brossard, N.; Osorio, F.; et al. Lipid-Encapsuled Grape Tannins Prevent Oxidative-Stress-Induced Neuronal Cell Death, Intracellular ROS Accumulation and Inflammation. Antioxidants 2022, 11, 1928. https://doi.org/10.3390/antiox11101928
Díaz HS, Ríos-Gallardo A, Ortolani D, Díaz-Jara E, Flores MJ, Vera I, Monasterio A, Ortiz FC, Brossard N, Osorio F, et al. Lipid-Encapsuled Grape Tannins Prevent Oxidative-Stress-Induced Neuronal Cell Death, Intracellular ROS Accumulation and Inflammation. Antioxidants. 2022; 11(10):1928. https://doi.org/10.3390/antiox11101928
Chicago/Turabian StyleDíaz, Hugo S., Angélica Ríos-Gallardo, Domiziana Ortolani, Esteban Díaz-Jara, María José Flores, Ignacio Vera, Angela Monasterio, Fernando C. Ortiz, Natalia Brossard, Fernando Osorio, and et al. 2022. "Lipid-Encapsuled Grape Tannins Prevent Oxidative-Stress-Induced Neuronal Cell Death, Intracellular ROS Accumulation and Inflammation" Antioxidants 11, no. 10: 1928. https://doi.org/10.3390/antiox11101928
APA StyleDíaz, H. S., Ríos-Gallardo, A., Ortolani, D., Díaz-Jara, E., Flores, M. J., Vera, I., Monasterio, A., Ortiz, F. C., Brossard, N., Osorio, F., & Río, R. D. (2022). Lipid-Encapsuled Grape Tannins Prevent Oxidative-Stress-Induced Neuronal Cell Death, Intracellular ROS Accumulation and Inflammation. Antioxidants, 11(10), 1928. https://doi.org/10.3390/antiox11101928