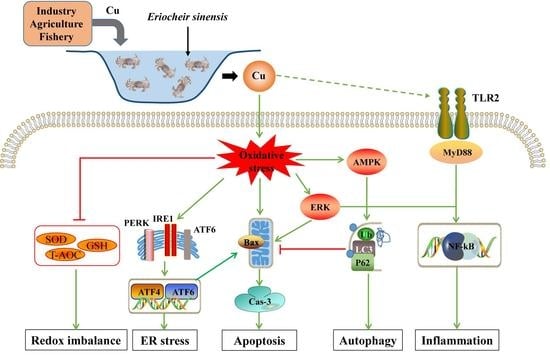
Effects of Copper Exposure on Oxidative Stress, Apoptosis, Endoplasmic Reticulum Stress, Autophagy and Immune Response in Different Tissues of Chinese Mitten Crab (Eriocheir sinensis)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crab Rearing, Experimental Design, and Sample Collection
2.2. Biochemical Assay
2.3. Quantitative Real-Time PCR Analysis
2.4. Integrated Biomarker Response Analysis
2.5. Statistical Analysis
3. Results
3.1. Alterations in the Redox State
3.2. Alterations in the Expression of Apoptosis-Related Genes
3.3. Alterations in the Expression of MAPK Pathway-Related Genes
3.4. Alterations in the Expression of ER Stress-Related Genes
3.5. Alterations in the Expression of Autophagy-Related Genes
3.6. Alterations in the Expression of Immune Response-Related Genes
3.7. Alterations in the Expression of Stress- and Detoxification-Related Genes
4. Discussion
4.1. Effects of Copper Exposure on Antioxidative Status
4.2. Effects of Copper Exposure on Apoptosis
4.3. Effects of Copper Exposure on ER Stress
4.4. Effects of Copper Exposure on Autophagy
4.5. Effects of Copper Exposure on the Immune Response
4.6. Effects of Copper Exposure on the Stress Response and Detoxification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tapiero, H.; Townsend, D.á.; Tew, K. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Ugarte, M.; Osborne, N.N.; Brown, L.A.; Bishop, P.N. Iron, zinc, and copper in retinal physiology and disease. Surv. Ophthalmol. 2013, 58, 585–609. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.J.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D.X. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- O’Gara, B.A.; Bohannon, V.K.; Teague, M.W.; Smeaton, M.B. Copper-induced changes in locomotor behaviors and neuronal physiology of the freshwater oligochaete, Lumbriculus variegatus. Aquat. Toxicol. 2004, 69, 51–66. [Google Scholar] [CrossRef]
- Tavares-Dias, M. Toxic, physiological, histomorphological, growth performance and antiparasitic effects of copper sulphate in fish aquaculture. Aquaculture 2021, 535, 736350. [Google Scholar] [CrossRef]
- Zhang, B.H.; Ding, Z.G.; Li, H.Q.; Mou, X.Z.; Zhang, Y.Q.; Yang, J.Y.; Zhou, E.M.; Li, W.J. Algicidal Activity of Streptomyces eurocidicus JXJ-0089 Metabolites and Their Effects on Microcystis Physiology. Appl. Environ. Microbiol. 2016, 82, 5132–5143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.Y.; Ju, Y.R.; Lin, C.J.; Tsai, J.W.; Chen, S.C.; Liao, C.M. Environmental stochasticity promotes copper bioaccumulation and bioenergetic response in tilapia. Stoch. Environ. Res. Risk Assess. 2015, 29, 1545–1555. [Google Scholar] [CrossRef]
- Zhang, F.; Li, D.; Yang, Y.; Zhang, H.; Zhu, J.; Liu, J.; Bu, X.; Li, E.; Qin, J.; Yu, N. Combined effects of polystyrene microplastics and copper on antioxidant capacity, immune response and intestinal microbiota of Nile tilapia (Oreochromis niloticus). Sci. Total Environ. 2022, 808, 152099. [Google Scholar] [CrossRef] [PubMed]
- Daglish, R.W.; Nowak, B.F. Rainbow Trout Gills Are a Sensitive Biomarker of Short-Term Exposure to Waterborne Copper. Arch. Environ. Contam. Toxicol. 2002, 43, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.; Dalton, R. The acute lethal toxicity to rainbow trout of mixtures of copper, phenol, zinc and nickel. J. Fish Biol. 1970, 2, 211–216. [Google Scholar] [CrossRef]
- Mohamed, M.; El-Fiky, S.; Soheir, Y.; Abeer, A. Cytogenetic studies on the effect of copper sulfate and lead acetate. pollution on Oreochromis niloticus fish. J. Cell Biol. 2008, 3, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Zang, W.; Yang, H.; Zhong, X.; Jiang, M.; Ke, X. The toxic effects of Cu2+,Zn2+,Cd2+ on giant freshwater prawn juvenile. J. Shanghai Fish. Univ. 2001, 10, 295–302. [Google Scholar] [CrossRef]
- Dong, X.; Lv, L.; Wang, A.; Wang, L.; Miao, J. Study on the acute toxicity of Cu2+ and Cd2+ acting on Procambarus clarkii juvenile. J. Hydroecology 2010, 3, 90–93. [Google Scholar] [CrossRef]
- Yao, Q.; Zang, W.l.; Dai, X.; Jiang, M.; Xu, G.; Ding, F. A acute toxic effects of copper, cadmium, dichlorvos and methamidophos on Penaeus vannamei larval shrimp and their interactions. J. Shanghai Fish. Univ. 2003, 12, 117–122. [Google Scholar]
- Tilton, F.A.; Bammler, T.K.; Gallagher, E.P. Swimming impairment and acetylcholinesterase inhibition in zebrafish exposed to copper or chlorpyrifos separately, or as mixtures. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Aardt, W.; Hough, M. Acute effects of Cu on oxygen consumption and 96 hr-LC50 values in the freshwater fish Tilapia sparrmani (Teleostei: Cichlidae) in Mooi River hard water, South Africa. Afr. J. Aquat. Sci. 2006, 31, 305–311. [Google Scholar] [CrossRef]
- Rougier, F.; Troutaud, D.; Ndoye, A.; Deschaux, P. Non-specific immune response of Zebrafish, Brachydanio rerio (Hamilton-Buchanan) following copper and zinc exposure. Fish Shellfish Immunol. 1994, 4, 115–127. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Hoseinifar, S.H.; Doan, H.V. Effect of dietary eucalyptol on stress markers, enzyme activities and immune indicators in serum and haematological characteristics of common carp (Cyprinus carpio) exposed to toxic concentration of ambient copper. Aquac. Res. 2018, 49, 3045–3054. [Google Scholar] [CrossRef]
- Vani Paila, R.; Rao Yallapragada, P.; Thatipaka, S.D.R. Response of glutathione system and carotenoids to sublethal copper in the postlarvae of Penaeus indicus. Ecotoxicol. Environ. Saf. 2012, 75, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Frías-Espericueta, M.G.; Castro-Longoria, R.; Barrón-Gallardo, G.J.; Osuna-López, J.I.; Abad-Rosales, S.M.; Páez-Osuna, F.; Voltolina, D. Histological changes and survival of Litopenaeus vannamei juveniles with different copper concentrations. Aquaculture 2008, 278, 97–100. [Google Scholar] [CrossRef]
- Tang, D.; Liu, R.; Shi, X.; Shen, C.; Bai, Y.; Tang, B.; Wang, Z. Toxic effects of metal copper stress on immunity, metabolism and pathologic changes in Chinese mitten crab (Eriocheir japonica sinensis). Ecotoxicology 2021, 30, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ge, X.; Hu, J.; Yu, H.; Jiang, Z. Effects of water-borne copper on the survival, antioxidant status, metallothionein-I mRNA expression and physiological responses of the Chinese mitten crab, Eriocheir sinensis (Decapoda: Brachyura) larvae. Sci. Mar. 2014, 78, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.B.; Zhao, Y.L.; Zhou, Z.; Yang, J. Effects of water CuSO4 concentration on molting, growth and survival of eriocheir sinensis. Acta Hydrobiol. Sin. 2006, 30, 563–569. [Google Scholar]
- Zhou, C. Toxic Effects of Copper Ions on Eriocheir sinensis; Nanjing Agricultural University: Nanjing, China, 2021. [Google Scholar]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; He, Q.; Xu, P.; Yin, G. Chronic exposure of hydrogen peroxide alters redox state, apoptosis and endoplasmic reticulum stress in common carp (Cyprinus carpio). Aquat. Toxicol. 2020, 229, 105657. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods–A Companion Methods Enzymol. 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, S. Study on Reciprocal Regulation Mechanism of MIH and Ec R Genes in the Molting Process of Chinese Mitten Crab, Eriocheir Sinensis; Shanghai Ocean University: Shanghai, China, 2017. [Google Scholar]
- Sanchez, W.; Burgeot, T.; Porcher, J.-M. A novel “Integrated Biomarker Response” calculation based on reference deviation concept. Environ. Sci. Pollut. Res. 2013, 20, 2721–2725. [Google Scholar] [CrossRef] [PubMed]
- Asih, A.Y.P.; Irawan, B.; Soegianto, A. Effect of copper on survival, osmoregulation, and gill structures of freshwater prawn (Macrobrachium rosenbergii, de Man) at different development stages. Mar. Freshw. Behav. Physiol. 2013, 46, 75–88. [Google Scholar] [CrossRef]
- Mukherjee, A. Experimental study of copper toxicity and some stress biomarkers in Macrobrachium scabriculum (Heller, 1862). J. Appl. Aquac. 2022, 34, 425–440. [Google Scholar] [CrossRef]
- Yeh, S.T.; Liu, C.H.; Chen, J.C. Effect of copper sulfate on the immune response and susceptibility to Vibrio alghiolyticus in the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2004, 17, 437–446. [Google Scholar] [CrossRef]
- Grosell, M.; Blanchard, J.; Brix, K.; Gerdes, R. Physiology is pivotal for interactions between salinity and acute copper toxicity to fish and invertebrates. Aquat. Toxicol. 2007, 84, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, Y.; Zhou, Z.; Zhou, X.; Yang, J. Effects of copper in water on distribution of copper and digestive enzymes activities in Eriocheir sinensis. J. Fish. CHINA 2005, 29, 496–501. [Google Scholar]
- Gurkan, M. Effects of three different nanoparticles on bioaccumulation, oxidative stress, osmoregulatory, and immune responses of Carcinus aestuarii. Toxicol. Environ. Chem. 2018, 100, 693–716. [Google Scholar] [CrossRef]
- Bremner, I. Manifestations of copper excess. Am. J. Clin. Nutr. 1998, 67, 1069S–1073S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Qian, D.; Xu, C.; Chen, C.; Qin, J.G.; Chen, L.; Li, E. Toxic effect of chronic waterborne copper exposure on growth, immunity, anti-oxidative capacity and gut microbiota of Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 100, 445–455. [Google Scholar] [CrossRef]
- Brouwer, M.; Brouwer, T.H. Biochemical defense mechanisms against copper-induced oxidative damage in the blue crab, Callinectes sapidus. Arch. Biochem. Biophys. 1998, 351, 257–264. [Google Scholar] [CrossRef]
- Ma’rifah, F.; Saputri, M.R.; Soegianto, A.; Irawan, B.; Putranto, T.W.C. The Change of Metallothionein and Oxidative Response in Gills of the Oreochromis niloticus after Exposure to Copper. Animals 2019, 9, 353. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.; Yang, J. Oxidative damage induced by copper and beta-cypermethrin in gill of the freshwater crayfish Procambarus clarkii. Ecotoxicol. Environ. Saf. 2015, 113, 446–453. [Google Scholar] [CrossRef]
- Capparelli, M.V.; Bordon, I.C.; Araujo, G.; Gusso-Choueri, P.K.; de Souza Abessa, D.M.; McNamara, J.C. Combined effects of temperature and copper on oxygen consumption and antioxidant responses in the mudflat fiddler crab Minuca rapax (Brachyura, Ocypodidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 223, 35–41. [Google Scholar] [CrossRef]
- Jiang, W.-D.; Liu, Y.; Hu, K.; Jiang, J.; Li, S.-H.; Feng, L.; Zhou, X.-Q. Copper exposure induces oxidative injury, disturbs the antioxidant system and changes the Nrf2/ARE (CuZnSOD) signaling in the fish brain: Protective effects of myo-inositol. Aquat. Toxicol. 2014, 155, 301–313. [Google Scholar] [CrossRef]
- Husak, V.V.; Mosiichuk, N.M.; Kubrak, O.I.; Matviishyn, T.M.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Acute exposure to copper induces variable intensity of oxidative stress in goldfish tissues. Fish Physiol. Biochem. 2018, 44, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Buettner, G.R. The EPR detection of lipid-dervied radicals during membrane lipid peroxidation of cells. Free Radic. Biol. Med. 1998, 25, S106. [Google Scholar] [CrossRef]
- Rajabiesterabadi, H.; Hoseini, S.M.; Fazelan, Z.; Hoseinifar, S.H.; Hien Van, D. Effects of dietary turmeric administration on stress, immune, antioxidant and inflammatory responses of common carp (Cyprinus carpio) during copper exposure. Aquac. Nutr. 2020, 26, 1143–1153. [Google Scholar] [CrossRef]
- Kong, X.; Jiang, H.; Wang, S.; Wu, X.; Fei, W.; Li, L.; Nie, G.; Li, X. Effects of copper exposure on the hatching status and antioxidant defense at different developmental stages of embryos and larvae of goldfish Carassius auratus. Chemosphere 2013, 92, 1458–1464. [Google Scholar] [CrossRef]
- Kamunde, C.; MacPhail, R. Effect of humic acid during concurrent chronic waterborne exposure of rainbow trout (Oncorhynchus mykiss) to copper, cadmium and zinc. Ecotoxicol. Environ. Saf. 2011, 74, 259–269. [Google Scholar] [CrossRef]
- Wei, K.; Yang, J. Copper-induced oxidative damage to the prophenoloxidase-activating system in the freshwater crayfish Procambarus clarkii. Fish Shellfish Immunol. 2016, 52, 221–229. [Google Scholar] [CrossRef]
- Wei, K.; Yang, J. Oxidative damage of hepatopancreas induced by pollution depresses humoral immunity response in the freshwater crayfish Procambarus clarkii. Fish Shellfish Immunol. 2015, 43, 510–519. [Google Scholar] [CrossRef]
- Santos, B.; Andrade, T.; Domingues, I.; Ribeiro, R.; Soares, A.M.V.M.; Lopes, I. Influence of salinity on the toxicity of copper and cadmium to Zebrafish embryos. Aquat. Toxicol. 2021, 241, 106003. [Google Scholar] [CrossRef]
- Carvalho, C.d.S.; Bernusso, V.A.; Fernandes, M.N. Copper levels and changes in pH induce oxidative stress in the tissue of curimbata (Prochilodus lineatus). Aquat. Toxicol. 2015, 167, 220–227. [Google Scholar] [CrossRef]
- AnvariFar, H.; Amirkolaie, A.K.; Jalali, A.M.; Miandare, H.K.; Sayed, A.H.; Üçüncü, S.İ.; Ouraji, H.; Ceci, M.; Romano, N. Environmental pollution and toxic substances: Cellular apoptosis as a key parameter in a sensible model like fish. Aquat. Toxicol. 2018, 204, 144–159. [Google Scholar] [CrossRef]
- Vergolyas, M.R.; Veyalkina, N.N.; Goncharuk, V.V. Effect of copper ions on hematological and cytogenetic parameters of freshwater fishes Carassius auratus gibelio. Cytol. Genet. 2010, 44, 124–128. [Google Scholar] [CrossRef]
- Luzio, A.; Monteiro, S.M.; Fontaínhas-Fernandes, A.; Pinto-Carnide, O.; Matos, M.; Coimbra, A.M. Copper induced upregulation of apoptosis related genes in zebrafish (Danio rerio) gill. Aquat. Toxicol. 2013, 128, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, K.X.; Wang, W.; Wang, C.G.; Shen, Y.C. Effects of Copper on Hemocyte Apoptosis, ROS Production, and Gene Expression in White Shrimp Litopenaeus vannamei. Biol. Trace Elem. Res. 2017, 179, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Samali, A.; Orrenius, S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000, 29, 323–333. [Google Scholar] [CrossRef]
- Hernandez, P.P.; Undurraga, C.; Gallardo, V.E.; Mackenzie, N.; Allende, M.L.; Reyes, A.E. Sublethal concentrations of waterborne copper induce cellular stress and cell death in zebrafish embryos and larvae. Biol. Res. 2011, 44, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Junttila, M.R.; Li, S.-P.; Westermarck, J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008, 22, 954–965. [Google Scholar] [CrossRef] [Green Version]
- Hiramatsu, N.; Kasai, A.; Yao, J.; Meng, Y.; Takeda, M.; Maeda, S.; Kitamura, M. AP-1-independent sensitization to oxidative stress-induced apoptosis by proteasome inhibitors. Biochem. Biophys. Res. Commun. 2004, 316, 545–552. [Google Scholar] [CrossRef]
- Nawaz, M.; Manzl, C.; Lacher, V.; Krumschnabel, G. Copper-induced stimulation of extracellular signal-regulated kinase in trout hepatocytes: The role of reactive oxygen species, Ca(2+), and cell energetics and the impact of extracellular signal-regulated kinase signaling on apoptosis and necrosis. Toxicol. Sci. 2006, 92, 464–475. [Google Scholar] [CrossRef]
- Zhang, C.H.; Wang, Y.; Sun, Q.Q.; Xia, L.L.; Hu, J.D.; Cheng, K.; Wang, X.; Fu, X.X.; Gu, H. Copper Nanoparticles Show Obvious in vitro and in vivo Reproductive Toxicity via ERK Mediated Signaling Pathway in Female Mice. Int. J. Biol. Sci. 2018, 14, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Schröder, M.; Kaufman, R.J. ER stress and the unfolded protein response. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 569, 29–63. [Google Scholar] [CrossRef]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Xu, Y.-C.; Hogstrand, C.; Zhao, T.; Wu, L.-X.; Zhuo, M.-Q.; Luo, Z. Waterborne copper exposure up-regulated lipid deposition through the methylation of GRP78 and PGC1α of grass carp Ctenopharyngodon idella. Ecotoxicol. Environ. Saf. 2020, 205, 111089. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-F.; Huang, C.; Shi, X.; Pan, Y.-X.; Liu, X.; Luo, Z. Endoplasmic reticulum stress and dysregulation of calcium homeostasis mediate Cu-induced alteration in hepatic lipid metabolism of javelin goby Synechogobius hasta. Aquat. Toxicol. 2016, 175, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-F.; Luo, Z.; Zhang, L.-H.; Hogstrand, C.; Pan, Y.-X. Endoplasmic reticulum stress and disturbed calcium homeostasis are involved in copper-induced alteration in hepatic lipid metabolism in yellow catfish Pelteobagrus fulvidraco. Chemosphere 2016, 144, 2443–2453. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, H.; Zhang, T.; Liu, J.-X. Copper induce zebrafish retinal developmental defects via triggering stresses and apoptosis. Cell Commun. Signal. 2020, 18, 45. [Google Scholar] [CrossRef] [Green Version]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Arencibia, M.G.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2011, 332, 1429. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.-C.; Zhao, T.; Hogstrand, C.; Chen, F.; Song, C.-C.; Luo, Z. Copper (Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J. Nutr. Biochem. 2022, 100, 108883. [Google Scholar] [CrossRef]
- Kang, Z.L.; Qiao, N.; Liu, G.Y.; Chen, H.M.; Tang, Z.X.; Li, Y. Copper-induced apoptosis and autophagy through oxidative stress-mediated mitochondrial dysfunction in male germ cells. Toxicol. Vitr. 2019, 61, 104639. [Google Scholar] [CrossRef]
- Li, Y.L.; Chen, H.M.; Liao, J.Z.; Chen, K.L.; Javed, M.T.; Qiao, N.; Zeng, Q.W.; Liu, B.X.; Yi, J.N.; Tang, Z.X.; et al. Long-term copper exposure promotes apoptosis and autophagy by inducing oxidative stress in pig testis. Environ. Sci. Pollut. Res. 2021, 28, 55140–55153. [Google Scholar] [CrossRef]
- Liao, J.Z.; Yang, F.; Chen, H.L.; Yu, W.L.; Han, Q.Y.; Li, Y.; Hu, L.M.; Guo, J.Y.; Pan, J.Q.; Liang, Z.P.; et al. Effects of copper on oxidative stress and autophagy in hypothalamus of broilers. Ecotoxicol. Environ. Saf. 2019, 185, 109710. [Google Scholar] [CrossRef]
- Luzio, A.; Parra, S.; Costa, B.; Santos, D.; Álvaro, A.; Monteiro, S. Copper impair autophagy on zebrafish (Danio rerio) gill epithelium. Environ. Toxicol. Pharmacol. 2021, 86, 103674. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.Z.; Yang, F.; Yu, W.L.; Qiao, N.; Zhang, H.; Han, Q.Y.; Hu, L.M.; Li, Y.; Guo, J.Y.; Pan, J.Q.; et al. Copper induces energy metabolic dysfunction and AMPK-mTOR pathway-mediated autophagy in kidney of broiler chickens. Ecotoxicol. Environ. Saf. 2020, 206, 111366. [Google Scholar] [CrossRef]
- Chen, H.L.; Wang, Y.Y.; Luo, J.; Kang, M.; Hou, J.; Tang, R.P.; Zhao, L.; Shi, F.; Ye, G.; He, X.L.; et al. Autophagy and apoptosis mediated nano-copper-induced testicular damage. Ecotoxicol. Environ. Saf. 2022, 229, 113039. [Google Scholar] [CrossRef] [PubMed]
- Fornai, F.; Puglisi-Allegra, S. Autophagy status as a gateway for stress-induced catecholamine interplay in neurodegeneration. Neurosci. Biobehav. Rev. 2021, 123, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.-J.; Zou, P.-F.; Yao, C.-L. Toll-like receptors (tlr) and its signaling pathway in teleost. Acta Hydrobiol. Sin. 2015, 39, 173–184. [Google Scholar]
- Wang, G.; Zhang, C.; Huang, B. Transcriptome analysis and histopathological observations of Geloina erosa gills upon Cr(VI) exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 231, 108706. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, B.W.; Koch, B.E.V.; Peijnenburg, W.J.G.M.; Vijver, M.G. Microbiota-dependent TLR2 signaling reduces silver nanoparticle toxicity to zebrafish larvae. Ecotoxicol. Environ. Saf. 2022, 237, 113522. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Sun, B.Z.; Wang, Z.Y. The crab Relish plays an important role in white spot syndrome virus and Vibrio alginolyticus infection. Fish Shellfish Immunol. 2019, 87, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Tang, Q.; Xia, Z.; Yi, S.; Cai, M.; Du, H.; Yang, J.; Li, J.; Xing, Q.; Luo, J.; et al. Molecular identification and functional analysis of MyD88 in giant freshwater prawn (Macrobrachium rosenbergii) and expression changes in response to bacterial challenge. Int. J. Biol. Macromol. 2021, 178, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, X.; Gao, X.; Jiang, Q.; Chen, Q.; Zhang, S.; Tong, S.; Liu, X.; Zhu, J.; Zhang, X. Pathogenicity of Aeromonas hydrophila causing mass mortalities of Procambarus clarkia and its induced host immune response. Microb. Pathog. 2020, 147, 104376. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.L.; Tian, X.; Nie, G.X.; Wang, J.L.; Liu, M.; Jiang, K.Y.; Wang, B.J.; Guo, Q.Q.; Huang, J.R.; Wang, L. The transcriptomic response to copper exposure in the digestive gland of Japanese scallops (Mizuhopecten yessoensis). Fish Shellfish Immunol. 2015, 46, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Aksakal, F.I.; Ciltas, A. Impact of copper oxide nanoparticles (CuO NPs) exposure on embryo development and expression of genes related to the innate immune system of zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 223, 78–87. [Google Scholar] [CrossRef]
- Park, J.-Y.; Chung, T.-W.; Jeong, Y.-J.; Kwak, C.-H.; Ha, S.-H.; Kwon, K.-M.; Abekura, F.; Cho, S.-H.; Lee, Y.-C.; Ha, K.-T. Ascofuranone inhibits lipopolysaccharide–induced inflammatory response via NF-kappaB and AP-1, p-ERK, TNF-α, IL-6 and IL-1β in RAW 264.7 macrophages. PLoS ONE 2017, 12, e0171322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Jia, Z.; Li, X.; Geng, X.; Sun, J. Identification and expression analysis of lipopolysaccharide-induced TNF-alpha factor gene in Chinese mitten crab Eriocheir sinensis. Fish Shellfish Immunol. 2014, 38, 190–195. [Google Scholar] [CrossRef]
- Tang, X.R.; Metzger, D.; Leeman, S.; Amar, S. LPS-induced TNF-alpha factor (LITAF)-deficient mice express reduced LPS-induced cytokine: Evidence for LITAF-dependent LPS signaling pathways. Proc. Natl. Acad. Sci. USA 2006, 103, 13777–13782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat shock proteins in toxicology: How close and how far? Life Sci. 2010, 86, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Reddy, M.M.; Mathur, N.; Saxena, D.K.; Chowdhuri, D.K. Induction of hsp70, hsp60, hsp83 and hsp26 and oxidative stress markers in benzene, toluene and xylene exposed Drosophila melanogaster: Role of ROS generation. Toxicol. Appl. Pharmacol. 2009, 235, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Y.; Guan, X.T.; Yao, L.L.; Zhang, H.; Jin, X.; Han, Y. Effects of Single and Joint Subacute Exposure of Copper and Cadmium on Heat Shock Proteins in Common Carp (Cyprinus carpio). Biol. Trace Elem. Res. 2016, 169, 374–381. [Google Scholar] [CrossRef]
- Yamuna, A.; Kabila, V.; Geraldine, P. Expression of heat shock protein 70 in freshwater prawn Macrobrachium malcolmsonii (H. Milne Edwards) following exposure to Hg and Cu. Indian J. Exp. Biol. 2000, 38, 921–925. [Google Scholar]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; He, Q.; Xu, P.; Yin, G. Immune, inflammatory, autophagic and DNA damage responses to long-term H2O2 exposure in different tissues of common carp (Cyprinus carpio). Sci. Total Environ. 2021, 757, 143831. [Google Scholar] [CrossRef]
- Amiard, J.C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.M.; Ball, J.S.; Williams, T.D.; Wu, H.; Ortega, F.; Van Aerle, R.; Katsiadaki, I.; Falciani, F.; Viant, M.R.; Chipman, J.K. Identifying health impacts of exposure to copper using transcriptomics and metabolomics in a fish model. Environ. Sci. Technol. 2010, 44, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, M.P.; Boyd, H.M.; Kelly, C.I.; Lete, I.R.; McLaughlin, Q.R. Modulation of endogenous antioxidants by zinc and copper in signal crayfish (Pacifastacus leniusculus). Chemosphere 2021, 275, 129982. [Google Scholar] [CrossRef] [PubMed]
- Uno, T.; Ishizuka, M.; Itakura, T. Cytochrome P450 (CYP) in fish. Environ. Toxicol. Pharmacol. 2012, 34, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Butt, A. Enzymatic and non-enzymatic detoxification in Lycosa terrestris and Pardosa birmanica exposed to single and binary mixture of copper and lead. Environ. Toxicol. Pharmacol. 2020, 80, 103500. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, K.-W. Identification and response of cytochrome P450 genes in the brackish water flea Diaphanosoma celebensis after exposure to benzo [α] pyrene and heavy metals. Mol. Biol. Rep. 2021, 48, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Luan, H.; Zhang, Y.; Wang, M.; Cao, D.; Yang, J.; Tang, J.; Fan, S.; Wu, X.; Hua, R. Interactive effects of diclofenac and copper on bioconcentration and multiple biomarkers in crucian carp (Carassius auratus). Chemosphere 2020, 242, 125141. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Devalraju, I.; Naqvi, N. Copper bioaccumulation and depuration by red swamp crayfish, Procambarus clarkii. Bull. Environ. Contam. Toxicol. 1998, 61, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Barron, M.G.; Adelman, I.R. Nucleic acid, protein content, and growth of larval fish sublethally exposed to various toxicants. Can. J. Fish. Aquat. Sci. 1984, 41, 141–150. [Google Scholar] [CrossRef]
- Lemus, M.; Chung, K. Effect of Temperature on Copper Toxicity, Accumulation, and Purification in Tropical Fish Juveniles Petenia kraussii (Pisces: Cichlidae). Caribb. J. Sci. 1999, 35, 64–69. [Google Scholar]
- Dan, Z.; Zhang, X.; Liu, D.; Ru, S. Cu accumulation, detoxification and tolerance in the red swamp crayfish Procambarus clarkii. Ecotoxicol. Environ. Saf. 2019, 175, 201–207. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; Su, S.; Song, C.; Yu, F.; Zhou, J.; Li, J.; Jia, R.; Xu, P.; Tang, Y. Effects of Copper Exposure on Oxidative Stress, Apoptosis, Endoplasmic Reticulum Stress, Autophagy and Immune Response in Different Tissues of Chinese Mitten Crab (Eriocheir sinensis). Antioxidants 2022, 11, 2029. https://doi.org/10.3390/antiox11102029
Feng W, Su S, Song C, Yu F, Zhou J, Li J, Jia R, Xu P, Tang Y. Effects of Copper Exposure on Oxidative Stress, Apoptosis, Endoplasmic Reticulum Stress, Autophagy and Immune Response in Different Tissues of Chinese Mitten Crab (Eriocheir sinensis). Antioxidants. 2022; 11(10):2029. https://doi.org/10.3390/antiox11102029
Chicago/Turabian StyleFeng, Wenrong, Shengyan Su, Changyou Song, Fan Yu, Jun Zhou, Jianlin Li, Rui Jia, Pao Xu, and Yongkai Tang. 2022. "Effects of Copper Exposure on Oxidative Stress, Apoptosis, Endoplasmic Reticulum Stress, Autophagy and Immune Response in Different Tissues of Chinese Mitten Crab (Eriocheir sinensis)" Antioxidants 11, no. 10: 2029. https://doi.org/10.3390/antiox11102029
APA StyleFeng, W., Su, S., Song, C., Yu, F., Zhou, J., Li, J., Jia, R., Xu, P., & Tang, Y. (2022). Effects of Copper Exposure on Oxidative Stress, Apoptosis, Endoplasmic Reticulum Stress, Autophagy and Immune Response in Different Tissues of Chinese Mitten Crab (Eriocheir sinensis). Antioxidants, 11(10), 2029. https://doi.org/10.3390/antiox11102029