Astaxanthin Protects against Hyperglycemia-Induced Oxidative and Inflammatory Damage to Bone Marrow and to Bone Marrow-Retained Stem Cells and Restores Normal Hematopoiesis in Streptozotocin-Induced Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
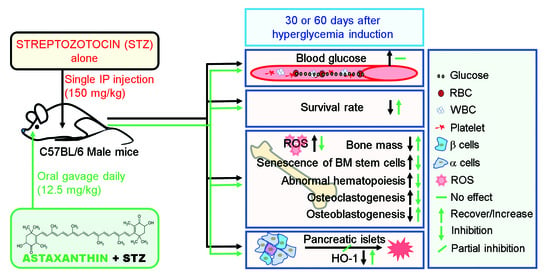
2.2. Animals, Treatment, and Sample Preparation
2.3. Micro-Computed Tomography (µCT) Analysis
2.4. Hematoxylin and Eosin (H&E) Staining of Trabecular Bone
2.5. Tartrate-Resistant Acid Phosphatase (TRAP) Staining of Femoral Bones
2.6. Immunohistochemistry (IHC)
2.7. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR) Assay
2.8. Flow Cytometric Analysis
2.9. Counting of Circulating Blood Cells
2.10. Measurement of RANKL in Serum
2.11. Ex Vivo and In Vitro Assays of BM-Derived Cells
2.11.1. Isolation and Culture of BMSCs and BMMs
2.11.2. Ex Vivo Assays to Assess BMSC Migration, Colony Formation, and Mineralization
2.11.3. Immunoblot Assay
2.11.4. TRAP Staining and qRT-PCR of BMMs
2.11.5. Assays for DNA Damage and Viability of Cultured BMMs
2.12. Statistical Analyses
3. Results
3.1. Oral Supplementation with ASTX Diminishes Severe Mortality, but Not Hyperglycemic Condition, in STZ-Induced Diabetic Mice
3.2. Oral Supplementation with ASTX Ameliorates the Impaired BM Microenvironment and Bone Mass Accrual in STZ-Induced Diabetic Mice
3.3. Long-Term Supplementation with ASTX Ameliorates STZ-Induced Formation of Osteoclasts and Production of RANKL
3.4. Long-Term Supplementation with ASTX Increases the Induction of Nrf2 and HO-1 in STZ-Induced Diabetic Mice
3.5. Supplemental ASTX Inhibits STZ-Induced Oxidative Stress and Senescence of BM HSCs and Recovers Hematopoietic Disorders in STZ-Injected Mice
3.6. Long-Term Supplementation with ASTX Suppresses STZ-Induced Complications in BM Retention and Senescence Induction of MSCs
3.7. Long-Term Hyperglycemia Disturbs Ang1-, SDF-1-, and Wnt-Associated Signaling Pathways in the BM
3.8. Long-Term Gavage of ASTX Recovers STZ-Induced Dysfunction of BMSC Migration, Colony Formation, and Mineralization
3.9. Direct Addition of ASTX Inhibits Osteoclastic Activation of BMMs in a Dose-Dependent Manner but Does Not Promote DNA Damage and Proliferation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muoio, D.M.; Newgard, C.B. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 3, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling and diabetes. Free. Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef] [PubMed]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of astaxanthin on diabetes pathogenesis and chronic complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajcyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and its effect on bone and fracture healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Marin, C.; Luyten, F.P.; Van der Schueren, B.; Kerckhofs, G.; Vandamme, K. The impact of type 2 diabetes on bone fracture healing. Front. Endocrinol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Orlandi, A.; Chavakis, E.; Seeger, F.; Tjwa, M.; Zeiher, A.M.; Dimmeler, S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic. Res. Cardiol. 2010, 105, 703–712. [Google Scholar] [CrossRef]
- Fadini, G.P.; Ferraro, F.; Quaini, F.; Asahara, T.; Madeddu, P. Concise review: Diabetes, the bone marrow niche, and impaired vascular regeneration. Stem Cells Transl. Med. 2014, 3, 949–957. [Google Scholar] [CrossRef]
- Kojima, H.; Kim, J.; Chan, L. Emerging roles of hematopoietic cells in the pathobiology of diabetic complications. Trends Endocrinol. Metab. 2014, 25, 178–187. [Google Scholar] [CrossRef]
- Chiba, H.; Ataka, K.; Iba, K.; Nagaishi, K.; Yamashita, T.; Fujimiya, M. Diabetes impairs the interactions between long-term hematopoietic stem cells and osteopontin-positive cells in the endosteal niche of mouse bone marrow. Am. J. Physiol. Cell. Physiol. 2015, 305, C693–C703. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, F.; Lymperi, S.; Méndez-Ferrer, S.; Saez, B.; Spencer, J.A.; Yeap, B.Y.; Masselli, E.; Graiani, G.; Prezioso, L.; Rizzini, E.L.; et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci. Transl. Med. 2011, 3, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Fadini, G.P.; Boscaro, E.; de Kreutzenberg, S.; Agostini, C.; Seeger, F.; Dimmeler, S.; Zeiher, A.; Tiengo, A.; Avogaro, A. Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care 2010, 33, 1097–1102. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [Green Version]
- Fadini, G.P.; Ciciliot, S.; Albiero, M. Concise review: Perspectives and clinical implications of bone marrow and circulating stem cell defects in diabetes. Stem Cells 2017, 35, 106–116. [Google Scholar] [CrossRef]
- Mercier, F.E.; Ragu, C.; Scadden, D.T. The bone marrow at the crossroads of blood and immunity. Nat. Rev. Immunol. 2012, 12, 49–60. [Google Scholar] [CrossRef]
- Motyl, K.; McCabe, L.R. Streptozotocin, type I diabetes severity and bone. Biol. Proceed. Online 2009, 11, 296–315. [Google Scholar] [CrossRef] [Green Version]
- Kook, S.H.; Yun, C.Y.; Sim, H.J.; Bhattarai, G.; Lee, B.C.; Lee, K.Y.; Cho, E.S.; Lee, J.C. Smad4 in osteoblasts exerts a differential impact on HSC fate depending on osteoblast maturation stage. Leukemia 2016, 30, 2039–2046. [Google Scholar] [CrossRef]
- Sugiura, M.; Nakamura, M.; Ikoma, Y.; Yano, M.; Ogawa, K.; Matsumoto, H.; Kato, M.; Ohshima, M.; Nagao, A. Serum carotenoid concentrations are inversely associated with serum aminotransferases in hyperglycemic subjects. Diabetes Res. Clin. Pract. 2006, 71, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Otton, R.; Marina, D.P.; Bolin, A.P.; dos Santos, R.C.M.; Polotow, T.G.; Sampaio, S.C.; de Barros, M.P. Astaxanthin ameliorates the redox imbalance in lymphocytes of experimental diabetic rats. Chem. Biol. Interact. 2010, 186, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hou, Y.; Li, J.; Wang, J. The role of astaxanthin on chronic diseases. Crystals 2021, 11, 505. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.G.; Fernandez, M.L. Potential of dietary non-provitamin A carotenoids in the prevention and treatment of diabetic microvascular complications. Adv. Nutr. 2016, 7, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H. Astaxanthin modulation of signaling pathways that regulate autophagy. Mar. Drugs 2019, 17, 546. [Google Scholar] [CrossRef] [Green Version]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a molecular therapeutic target for astaxanthin. Biomed. Pharmacother. 2021, 137, 111374. [Google Scholar] [CrossRef]
- Bhattarai, G.; So, H.S.; Kieu, T.T.T.; Kook, S.H.; Lee, J.C.; Jeon, Y.M. Astaxanthin inhibits diabetes-triggered periodontal destruction, ameliorates oxidative complications in STZ-injected mice, and recovers Nrf2-dependent antioxidant system. Nutrients 2021, 13, 3575. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Gross, M. Effect of carotenoids on in vitro immunoglobulin production by human peripheral blood mononuclear cells: Astaxanthin, a carotenoid without vitamin A activity, enhances in vitro immunoglobulin production in response to a T-dependent stimulant and antigen. Nutr. Cancer 1995, 23, 171–183. [Google Scholar] [CrossRef]
- Poudel, S.B.; So, H.S.; Sim, H.J.; Cho, J.S.; Cho, E.S.; Jeon, Y.M.; Kook, S.H.; Lee, J.C. Osteoblastic Wntless deletion differentially regulates the fate and functions of bone marrow-derived stem cells in relation to age. Stem Cells 2021, 39, 103–114. [Google Scholar] [CrossRef]
- Kiel, M.J.; Yilmaz, O.H.; Iwashita, T.; Yilmaz, O.H.; Terhorst, C.; Morrison, S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005, 121, 1109–1121. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Wang, Y.; Wang, J.; Zhai, J.; He, F.; Zhu, G. Irradiation-induced senescence of bone marrow mesenchymal stem cells aggravates osteogenic differentiation dysfunction via paracrine signaling. Am. J. Physiol. Cell Physiol. 2020, 318, C1005–C1017. [Google Scholar] [CrossRef]
- Zhuge, F.; Ni, Y.; Wan, C.; Liu, F.; Fu, Z. Anti-diabetic effects of astaxanthin on an STZ-induced diabetic model in rats. Endocr. J. 2021, 68, 451–459. [Google Scholar] [CrossRef]
- Lu, H.; Kraut, D.; Gerstenfeld, L.C.; Graves, D.T. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 2003, 144, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Luis, T.C.; Naber, B.A.; Roozen, P.P.; Brugman, M.H.; de Haas, E.F.; Ghazvini, M.; Fibbe, W.E.; van Dongen, J.J.; Fodde, R.; Staal, F.J. Canonical Wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell 2011, 9, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Verovskaya, E.; de Haan, G. Noncanonical Wnt comes of age in hematopoietic stem cells. Cell Stem Cell 2013, 13, 642–643. [Google Scholar] [CrossRef] [Green Version]
- Lidia, I.; Ferrándiz, M.L.; Brines, R.; Guede, D.; Cuadrado, A.; Alcaraz, M.J. Effects of Nrf2 deficiency on bone microarchitecture in an experimental model of osteoporosis. Oxid. Med. Cell. Longev. 2014, 2014, 1–10. [Google Scholar]
- Sima, C.; Aboodi, G.M.; Lakschevitz, F.S.; Sun, C.; Goldberg, M.B.; Glogauer, M. Nuclear factor erythroid 2-related factor 2 down-regulation in oral neutrophils is associated with periodontal oxidative damage and severe chronic periodontitis. Am. J. Pathol. 2016, 186, 1417–1426. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yu, H.; Pan, H.; Zhou, X.; Ruan, Q.; Kong, D.; Chu, Z.; Li, H.; Huang, J.; Huang, X.; et al. Nrf2 suppression delays diabetic wound healing through sustained oxidative stress and inflammation. Front. Pharmacol. 2019, 10, 1099. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Luo, J.; Jing, X.; Guo, J.; Yao, X.; Hao, X.; Ye, Y.; Liang, S.; Lin, J.; Wang, G.; et al. Astaxanthin protects against osteoarthritis via Nrf2: A guardian of cartilage homeostasis. Aging 2019, 11, 10513. [Google Scholar] [CrossRef] [PubMed]
- Fleming, H.E.; Janzen, V.; Lo Celso, C.; Guo, J.; Leahy, K.M.; Kronenberg, H.M.; Scadden, D.T. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2008, 2, 274–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reya, T.; Duncan, A.W.; Ailles, L.; Domen, J.; Scherer, D.C.; Willert, K.; Hintz, L.; Nusse, R.; Weissman, I.L. A role for Wnt signaling in self-renewal of haematopoietic stem cells. Nature 2003, 423, 409–414. [Google Scholar] [CrossRef]
- Nusse, R. Wnt signaling in disease and in development. Cell Res. 2005, 15, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Saunders, T.L.; Enikolopov, G.; Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012, 481, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Frenette, P.S. Niches for hematopoietic stem cells and their progeny. Immunity 2018, 48, 632–648. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.L.; Han, X.D.; Li, Y.; Chu, X.F.; Miao, W.M.; Zhang, J.L.; Fan, S.J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. Ther. 2017, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Yan, X.; Wintergerst, K.A.; Cai, L.; Keller, B.B.; Tan, Y. Nrf2: Redox and metabolic regulator of stem cell state and function. Trends Mol. Med. 2020, 26, 185–200. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattarai, G.; So, H.-S.; Kim, T.-G.; Kieu, T.T.T.; Kim, Y.-W.; Yang, K.-R.; Lee, J.-C.; Kook, S.-H.; Jeon, Y.-M. Astaxanthin Protects against Hyperglycemia-Induced Oxidative and Inflammatory Damage to Bone Marrow and to Bone Marrow-Retained Stem Cells and Restores Normal Hematopoiesis in Streptozotocin-Induced Diabetic Mice. Antioxidants 2022, 11, 2321. https://doi.org/10.3390/antiox11122321
Bhattarai G, So H-S, Kim T-G, Kieu TTT, Kim Y-W, Yang K-R, Lee J-C, Kook S-H, Jeon Y-M. Astaxanthin Protects against Hyperglycemia-Induced Oxidative and Inflammatory Damage to Bone Marrow and to Bone Marrow-Retained Stem Cells and Restores Normal Hematopoiesis in Streptozotocin-Induced Diabetic Mice. Antioxidants. 2022; 11(12):2321. https://doi.org/10.3390/antiox11122321
Chicago/Turabian StyleBhattarai, Govinda, Han-Sol So, Tae-Geum Kim, Thi Thu Trang Kieu, Yeon-Woo Kim, Ku-Ri Yang, Jeong-Chae Lee, Sung-Ho Kook, and Young-Mi Jeon. 2022. "Astaxanthin Protects against Hyperglycemia-Induced Oxidative and Inflammatory Damage to Bone Marrow and to Bone Marrow-Retained Stem Cells and Restores Normal Hematopoiesis in Streptozotocin-Induced Diabetic Mice" Antioxidants 11, no. 12: 2321. https://doi.org/10.3390/antiox11122321
APA StyleBhattarai, G., So, H. -S., Kim, T. -G., Kieu, T. T. T., Kim, Y. -W., Yang, K. -R., Lee, J. -C., Kook, S. -H., & Jeon, Y. -M. (2022). Astaxanthin Protects against Hyperglycemia-Induced Oxidative and Inflammatory Damage to Bone Marrow and to Bone Marrow-Retained Stem Cells and Restores Normal Hematopoiesis in Streptozotocin-Induced Diabetic Mice. Antioxidants, 11(12), 2321. https://doi.org/10.3390/antiox11122321