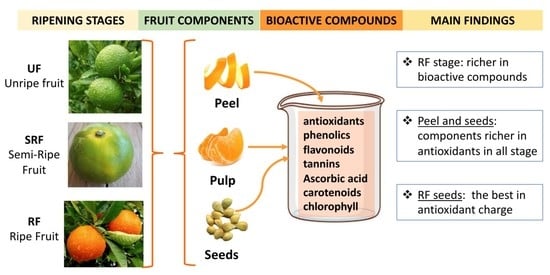
Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. Photosynthetic Pigments Content Analysis
2.3. Phenolic Extraction
2.4. Determination of Total Polyphenols
2.5. Flavonoid Content and Total Condensed Tannins
2.6. Ascorbic Acid Content
2.7. Antioxidant Activity Assays
2.7.1. FRAP Assay
2.7.2. DPPH Assay
2.8. Characterization of Phenolic Compounds in Peel and Seeds by Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.8.1. Preparation of Standard Solutions and Samples
2.8.2. LC-MS/MS Instrumentation and Conditions
2.8.3. Quantification of Analytes
2.9. Statistical Analysis
3. Results
3.1. Total Phenolic Compounds
3.2. Ascorbic Acid
3.3. Sample Antioxidant Activity
3.4. Total Chlorophyll and Carotenoid Content
3.5. Characterization of Phenolic Compounds in Peel and Seeds at Diverse Ripening Stages
3.6. Heatmap Analysis
3.7. Interaction between Fruit Component and Ripening Stages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Muller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Patil, B.S.; Jayaprakasha, G.K.; Murthy, K.N.C.; Vikram, A. Bioactive compounds: Historical perspectives, opportunities, and challenges. J. Agric. Food Chem. 2009, 57, 8142–8160. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, H.; Li, G.X.; Yang, Z.; Guan, F.; Jin, H. Cancer prevention by tea: Evidence from laboratory studies. Pharmacol. Res. 2011, 64, 113–122. [Google Scholar] [CrossRef]
- Barden, L.; Decker, E.A. Lipid Oxidation in Low-moisture Food: A Review. Food Sci. Nutr. 2016, 56, 2467–2482. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Heying, E.; Tanumihardjo, S.A. History, Global Distribution, and Nutritional Importance of Citrus Fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Tounsi, M.S. Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind. Crop. Prod. 2012, 39, 74–80. [Google Scholar] [CrossRef]
- Rapisarda, P.L.; Bianco, M.; Pannuzzo, P.; Timpanaro, N. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Postharvest Biol. Tec. 2008, 49, 348–354. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar] [PubMed]
- Peterson, J.J.; Dwyera, J.T.; Beecherb, G.R.; Bhagwat, S.A.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: A compilation and review of the data from the analytical literature. J. Food Compos. Anal. 2006, 19, 66–73. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Girennavar, B.; Patil, B.S. Radical scavenging activities of rio red grapefruits and sour orange fruit extracts in different in vitro model systems. Bioresour. Technol. 2008, 99, 4484–4494. [Google Scholar] [CrossRef]
- Kelebek, H.; Canbas, A.; Selli, S. Determination of phenolic composition and antioxidant capacity of blood orange juices obtained from cvs. Moro and Sanguinello (Citrus sinensis (L.) Osbeck) grown in Turkey. Food Chem. 2008, 107, 1710–1716. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid composition and antioxidant activity of juices from chinotto (Citrus myrtifolia Raf.) fruits at different ripening stages. J. Agric. Food Chem. 2010, 58, 3031–3036. [Google Scholar]
- Ramful, D.; Bahorun, T.; Bourdon, E.; Tarnus, E.; Aruoma, O.I. Bioactive phenolics and antioxidant propensity of flavedo extracts of Mauritian citrus fruits: Potential prophylactic ingredients for functional foods application. Toxicology 2010, 278, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmentally friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Rezzadori, K.; Benedetti, S.; Amante, E.R. Proposals for the residues recovery: Orange waste as raw material for new products. Food Bioprod. Process. 2012, 90, 606–614. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Bampidis, V.A.; Robinson, P.H. Citrus by-products as ruminant feeds: A review. Anim. Feed Sci. Technol. 2006, 128, 175–217. [Google Scholar] [CrossRef]
- Hayat, K.; Zhang, X.M.; Chen, H.Q.; Xia, S.Q.; Jia, C.S.; Zhong, F. Liberation and separation of phenolic compounds from citrus mandarin peels by microwave heating and its effect on antioxidant activity. Sep. Purif. Technol. 2010, 73, 371–376. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Londono-Londono, J.; de Lima, V.R.; Lara, O.; Gil, A.; Pasa, T.B.C. Clean recovery of antioxidant flavonoids from citrus peel: Optimizing an aqueous ultrasound-assisted extraction method. Food Chem. 2010, 119, 81–87. [Google Scholar] [CrossRef]
- Sun, Y.S.; Wang, J.H.; Gu, S.B.; Liu, Z.B.; Zhang, Y.J.; Zhang, X.X. Simultaneous determination of flavonoids in different parts of citrus reticulata ‘Chachi’ fruit by high performance liquid chromatography-photodiode array detection. Molecules 2010, 15, 5378–5388. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, G.; Iesce, M.R.; Naviglio, D.; Ciaravolo, M.; Vitale, E.; Arena, C. Comparative studies on different citrus cultivars: A revaluation of waste mandarin components. Antioxidants 2020, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Maduwanthi, S.D.T.; Marapana, R.A.U.J. Induced Ripening Agents and Their Effect on Fruit Quality of Banana. Int. J. Food Sci. 2019, 2019, 2520179. [Google Scholar] [CrossRef] [PubMed]
- Alquezar, B.; Rodrigo, M.J.; Zacarias, L. Carotenoid biosynthesis and their regulation in citrus fruits. Tree For. Sci. Biotech. 2008, 23–35. [Google Scholar]
- Multari, S.; Licciardello, C.; Caruso, M.; Martens, S. Monitoring the changes in phenolic compounds and carotenoids occurring during fruit development in the tissues of four citrus fruits. Food Res. Int. 2020, 134, 109–228. [Google Scholar] [CrossRef]
- Wikandari, R.; Nguyen, H.; Millati, R.; Niklasson, C.; Taherzadeh, M.J. Improvement of biogas production from orange peel waste by leaching of limonene. BioMed Res. Int. 2015, 49, 41–82. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Pontoni, L.; Porqueddu, I.; Greco, R.; Pirozzi, F.; Malpei, F. Effect of the concentration of essential oil on orange peel waste biomethanization: Preliminary batch results. Waste Manag. 2016, 48, 440–447. [Google Scholar] [CrossRef]
- Deng, G.F.; Shen, C.; Xu, X.R.; Kuang, R.D.; Guo, Y.J.; Zeng, L.S.; Gao, L.-L.; Lin, X.; Xie, J.-F.; Xia, E. Potential of fruit wastes as natural resources of bioactive compounds. Int. J. Mol. Sci. 2012, 13, 8308–8323. [Google Scholar] [CrossRef] [Green Version]
- Manthey, J.A.; Grohmann, K. Phenols in citrus peel by products: Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J. Agric. Food Chem. 2001, 49, 3268–3273. [Google Scholar] [CrossRef]
- Elkhatim, K.A.S.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Sun, B.; Richardo-da-Silva, J.M.; Sprenger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Arena, C.; Vitale, L.; Bianchi, A.R.; Mistretta, C.; Vitale, E.; Parisi, C.; Guerriero, G.; Magliulo, V.; De Maio, A. The ageing process affects the antioxidant defences and the poly (ADPribosyl)ation activity in Cistus incanus L. leaves. Antioxidants 2019, 8, 528. [Google Scholar] [CrossRef] [Green Version]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays et al. Determination of Antioxidant Capacity. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Ramful, D.; Tarnus, E.; Aruoma, O.; Bourdon, E.; Bahorun, T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Int. Food Res. J. 2011, 44, 2088–2099. [Google Scholar] [CrossRef]
- Bashir, H.A.; Abu-Goukh, A. Compositional changes during guava fruit ripening. Food Chem. 2003, 80, 557–563. [Google Scholar] [CrossRef]
- Vendramini, A.L.; Turgo, L.C. Chemical composition of Acerola fruit (Malpighia punicifolia L.) at three stages of maturity. Food Chem. 2000, 71, 195–198. [Google Scholar] [CrossRef]
- Caggia, C.; Palmeri, R.; Russo, N.; Timpone, R.; Randazzo, C.L.; Todaro, A.; Barbagallo, S. Employ of Citrus By-product as Fat Replacer Ingredient for Bakery Confectionery Products. Front. Nutr. 2020, 7, 46. [Google Scholar] [CrossRef]
- Racchi, M.L. Antioxidant Defenses in Plants with Attention to Prunus and Citrus spp. Antioxidants 2013, 2, 340–369. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Casals, B.A.; Byrne, D.; Okie, W.R.; Cisneros-Zevallos, L. Selecting new peach and plum genotypes rich in phenolic compounds and enhanced functional properties. Food Chem. 2006, 96, 273–280. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC-DAD-ESIMS analysis of phenolic compounds in nectarines, peaches and plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Int. Food Res. J. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Kondo, S.; Tsuda, K.; Muto, N.; Ueda, J. Antioxidative activity of apples skin or flesh extracts associated with fruit development on selected apple cultivars. Sci. Hortic. 2002, 96, 177–185. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.; Bonesi, R.; Menichini, M.; Curr, F. Potential role of natural compounds against skin aging. Med. Chem. 2015, 22, 1515–1538. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, J.P. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm. f. BMC Res. Notes 2017, 10, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Ou-Lee, T.M.; Raba, R.; Amundson, R.G.; Last, R.L. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 1993, 5, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Feeny, P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 1970, 51, 565–581. [Google Scholar] [CrossRef]
- Dalla Valle, A.Z.; Mignani, I.; Spinardi, A.; Galvano, F.; Ciappellano, S. The antioxidant profile of three different peaches cultivars (Prunus persica) and their short-term effect on antioxidant status in human. Eur. Food Res. Technol. 2007, 225, 167–172. [Google Scholar] [CrossRef]
- Vázquez-Gutiérrez, J.L.; Quiles, A.; Hernando, I.; Pérez-Munuera, I. Changes in the microstructure and location of some bioactive compounds in persimmons treated by high hydrostatic pressure. Postharvest Biol. Technol. 2011, 61, 137–144. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity of fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stages. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M.I. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J. Food Comp. Anal. 2006, 19, 544–551. [Google Scholar] [CrossRef]
- Kondakova, V.; Tsvetkov, I.; Batchvarova, R.; Badjakov, I.; Dzhambazova, T.; Slavov, S. Phenol compounds—Qualitative index in small fruits. Biotechnol. Biotech. Eq. 2009, 23, 1444–1448. [Google Scholar] [CrossRef] [Green Version]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lila, M.A. Anthocyanins and human health: An in vitro investigative approach. J. Biomed. Biotechnol. 2004, 5, 306–313. [Google Scholar] [CrossRef]
- Sari, D.R.T.; Cairns, J.R.K.; Safitri, A.; Fatchiyah, F. Virtual prediction of the Delphinidin-3-O glucoside and Peonidin-3-O-glucoside as anti-inflammatory of TNF-α signaling. Acta Inform. Med. 2019, 27, 152–157. [Google Scholar] [CrossRef]
- Ramalingayya, G.V.; Nampoothiri, M.; Nayak, P.G.; Kishore, A.; Shenoy, R.R.; Rao, C.M.; Nandakumar, K. Naringin and Rutin Alleviates Episodic Memory Deficits in Two Differentially Challenged Object Recognition Tasks. Pharmacogn. Mag. 2016, 12, 63–70. [Google Scholar]
- Smith, M.; Smith, J.C. Repurposing therapeutics for COVID-19: Supercomputer-based docking to the SARS-CoV-2 viral spike protein and viral spike protein-human ACE2 interface. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Jain, V.; Viswanatha, G.L.; Manohar, D.; Shivaprasad, H.N. Isolation of Antidiabetic Principle from Fruit Rinds of Punica granatum. Evid. Based Complement. Altern. Med. 2012, 2012, 147202. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L. Effects of Vitamin C on health: A review of evidence. Front. Biosci. 2013, 18, 1017–1029. [Google Scholar]
- Fattahi, J.; Hamidoghli, Y.; Ghazvini, R.F.; Ghasemnejad, M.; Bakhshi, D. Assessment of fruit quality and antioxidant activity of three citrus species during ripening. South-West. J. Hortic. Biol. Environ. 2011, 2, 113–128. [Google Scholar]
- Jimenez, A.; Cressen, G.; Kular, B.; Firmin, j.; Robinson, S.; Verhoeyen, M.; Mullineaux, P. Changes in oxidative process and components of the antioxidant system during tomato fruit ripening. Planta 2002, 214, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Agócs, A.; Nagy, V.; Szabó, Z.; Márk, L.; Ohmacht, R.; Deli, J. Comparative study on the carotenoid composition of the peel and the pulp of different citrus species. Innov. Food Sci. Emerg. Technol. 2007, 8, 390–394. [Google Scholar] [CrossRef]
- Charoenchongsuk, N.; Matsumoto, D.; Itai, A.; Murayama, H. Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP. Horticulturae 2018, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Seifert, B.; Pflanz, M.; Zude, M. Spectral shift as advanced index for fruit chlorophyll breakdown. Food Bioproc. Technol. 2013, 7, 2050–2059. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Xu, X.; Zhou, X.; Liu, Y.; Zhao, Z. Changes in phenolic profiles and antioxidant activity in rabbiteye blueberries during ripening. Int. J. Food Prop. 2019, 22, 320–329. [Google Scholar]
- Rascio, N.; Casadoro, G. Cotyledon greening in hypogeal seeds of citrus fruits. J. Ultrastruct. Mol. Struct. Res. 1988, 101, 13–22. [Google Scholar] [CrossRef]
- Maluf, M.P.; Saab, I.N.; Wurtzel, E.T.; Sachs, M.M. The viviparous 12 maize mutant is deficient in abscisic acid, carotenoids, and chlorophyll synthesis. J. Exp. Bot. 1997, 48, 1259–1268. [Google Scholar] [CrossRef] [Green Version]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005; p. 260. [Google Scholar] [CrossRef]
- Calucci, L.; Capocchi, A.; Galleschi, L.; Ghiringhelli, S.; Pinzino, C.; Saviozzi, F.; Zandomeneghi, M. Antioxidants, free radicals, storage proteins, puroindolines, and proteolytic activities in bread wheat (Triticum aestivum) seeds during accelerated aging. J. Agric. Food Chem. 2004, 52, 4274–4281. [Google Scholar] [CrossRef]
- Zakynthinos, G.; Varzakas, T. Carotenoids: From plants to food industry. Curr. Res. Nutr. Food Sci. 2016, 4, 38–51. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Lee, J.G. Ripening-dependent changes in antioxidants, color attributes, and antioxidant activity of seven tomato (Solanum lycopersicum L.) cultivars. J. Anal. Methods Chem. 2016, 2016, 5498618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouglias, N.; Papachattzis, A.; Lazou, E.; Tsiountsioura, V.; Ntalla, M.N. Effect of ripening stage on the total phenolics content, lycopene and antioxidant activity of tomato fruits grown to a geothermal greenhouse. Ann. Univ. Craiova Ser. Biol. Hortic. Food Prod. Process. Technol. Environ. Eng. 2018, 23, 115–120. [Google Scholar]
- Hegedus, A.; Pfeiffer, P.; Papp, N.; Abranko, L.; Blazovics, A.; Pedryc, A.; Stefanovits-Banyai, E. Accumulation of antioxidants in apricot fruit through ripening: Characterization of a genotype with enhanced functional properties. Biol. Res. 2011, 44, 339–344. [Google Scholar] [CrossRef]





| Compounds | UF (µg L−1) | SRF (µg L−1) | RF (µg L−1) |
|---|---|---|---|
| Delphinidin-3,5-diglucoside | 3.07 ± 0.20 | 4.14 ± 0.22 | 1.84 ± 0.09 |
| Cyanidin-3,5-di-O-glucoside | 2.87 ± 0.11 | 2.26 ± 0.14 | 3.16 ± 0.14 |
| Delphinidin-3-O-glucoside | 2032.45 ± 177.38 | 2644.42 ± 96.01 | 2102.92 ± 115.58 |
| Cyanidin-3-O-glucoside | 154.87 ± 8.48 | 161.96 ± 11.32 | 88.26 ± 3.04 |
| Delphinidin-3-O-arabinoside | 8.55 ± 0.30 | 6.21 ± 0.61 | 2.49 ± 0.10 |
| Petunidin-3-O-glucoside | 60.47 ± 4.32 | 53.93 ± 4.76 | 53.80 ± 2.95 |
| Cyanidin-3-O-arabinoside | 1.92 ± 0.14 | 1.15 ± 0.07 | - |
| Pelargonidin-3-O-glucoside | 75.07 ± 3.21 | 53.23 ± 1.83 | 27.84 ± 1.14 |
| Peonidin-3-O-glucoside | 143.74 ± 19.28 | 199.68 ± 10.57 | 339.94 ± 6.79 |
| Malvidin-3-O-glucoside | 82.20 ± 3.65 | 70.21 ± 5.07 | 86.93 ± 4.52 |
| Malvidin-3-O-arabinoside | 18.34 ± 1.19 | 22.43 ± 0.79 | 9.79 ± 0.43 |
| Delphinidin rutinoside | 4019.47 ± 304.32 | 4407.85 ± 266.85 | 3255.32 ± 219.63 |
| Malvidin 3-O-p-coumaroylglucoside | 16.96 ± 0.80 | 11.59 ± 0.44 | 17.41 ± 0.36 |
| Naringin | 391.61 ± 20.89 | 445.15 ± 26.70 | 40.56 ± 3.12 |
| Quercetin-3-glucoside | 2083.89 ± 104.38 | 2654.77 ± 144.51 | 2048.06 ± 119.97 |
| EGC 3-gallate | - | 6.26 ± 0.52 | 10.33 ± 0.46 |
| GC 3-gallate | - | 4.53 ± 0.50 | 9.50 ± 0.71 |
| Gallic acid | 52.09 ± 2.09 | 42.42 ± 2.21 | 47.66 ± 2.42 |
| Syringaldehyde | 3.30 ± 0.12 | 5.19 ± 0.45 | 9.69 ± 0.43 |
| Syringic acid | 32.83 ± 1.14 | 20.91 ± 1.23 | 41.64 ± 2.48 |
| Chlorogenic acid | 68.90 ± 5.43 | 81.74 ± 4.25 | 53.68 ± 1.45 |
| Quercetin-3-O-rhamnoside | 76.12 ± 4.04 | 76.99 ± 1.15 | 56.09 ± 4.65 |
| Valoneic acid dilactone | 1430.20 ± 68.58 | 103.41 ± 12.63 | 268.53 ± 10.98 |
| Phloretin | 9.77 ± 0.60 | 8.66 ± 0.58 | 9.68 ± 0.83 |
| Phloridzin | 19.75 ± 0.74 | 20.90 ± 2.47 | 19.51 ± 0.80 |
| Myricitrin | 137.26 ± 11.79 | 83.68 ± 8.06 | 83.54 ± 1.89 |
| Sinensetin | 4164.48 ± 265.24 | 2024.25 ± 228.68 | 1769.17 ± 117.64 |
| Rutin | 3608.01 ± 175.96 | 4055.79 ± 207.04 | 3610.47 ± 222.90 |
| Compounds | UF (µg L−1) | SRF (µg L−1) | RF (µg L−1) |
|---|---|---|---|
| Delphinidin diglucoside | 0.36 ± 0.02 | - | - |
| Cyanidin-3,5-di-O-glucoside | 14.07 ± 0.86 | 35.19 ± 1.85 | 22.75 ± 0.92 |
| Delphinidin-3-O-glucoside | 66.61 ± 7.17 | 19.76 ± 2.06 | 11.27 ± 0.76 |
| Cyanidin-3-O-glucoside | 174.57 ± 6.46 | 293.95 ± 8.49 | 345.78 ± 22.55 |
| Delphinidin-3-O-arabinoside | 15.38 ± 1.42 | 5.25 ± 0.26 | 9.69 ± 0.35 |
| Petunidin-3-O-glucoside | 40.81 ± 6.08 | 16.91 ± 0.78 | 24.25 ± 2.92 |
| Cyanidin-3-O-arabinoside | 7.07 ± 0.47 | 9.31 ± 0.50 | 23.97 ± 1.65 |
| Pelargonidin-3-O-glucoside | 54.79 ± 4.01 | 35.73 ± 3.11 | 40.58 ± 1.41 |
| Peonidin-3-O-glucoside | 34.72 ± 2.20 | - | 14.09 ± 1.05 |
| Malvidin-3-O-glucoside | 2.15 ± 0.35 | - | 4.64 ± 0.50 |
| Malvidin-3-O-arabinoside | 3.21 ± 0.12 | - | - |
| Delphinidin rutinoside | 56.16 ± 3.33 | 16.14 ± 1.24 | 3.83 ± 0.11 |
| Malvidin 3-O-p-coumaroylglucoside | 2.51 ± 0.09 | - | - |
| Naringin | 15.31 ± 1.07 | 3.03 ± 0.13 | 4.31 ± 0.20 |
| Quercetin-3-glucoside | 40.21 ± 2.20 | 15.56 ± 1.11 | 6.14 ± 0.35 |
| Procyanidin B1 | 6.61 ± 0.90 | - | - |
| Catechin | 0.67 ± 0.07 | 11.09 ± 0.79 | 8.74 ± 0.69 |
| Epicatechin | 1.86 ± 0.16 | 1.59 ± 0.10 | 7.22 ± 0.25 |
| Catechin-3-gallate | 12.35 ± 0.57 | 18.46 ± 0.98 | 14.93 ± 0.73 |
| EC-3-gallate | 12.40 ± 1.58 | 16.59 ± 0.83 | 12.44 ± 1.46 |
| EGC 3-gallate | 21.74 ± 1.18 | 9.08 ± 0.94 | 8.03 ± 0.33 |
| GC 3-gallate | 21.29 ± 0.71 | 18.10 ± 0.66 | 9.26 ± 0.55 |
| Gallic acid | 71.80 ± 5.73 | 66.27 ± 3.43 | 28.93 ± 1.46 |
| Syringaldehyde | 6.82 ± 0.42 | 0.69 ± 0.02 | - |
| Syringic acid | 40.33 ± 2.63 | 18.85 ± 0.63 | 12.11 ± 1.28 |
| 6-Malonyldaidzin | 109.00 ± 5.60 | 67.11 ± 4.24 | 143.32 ± 14.93 |
| Chlorogenic acid | 44.56 ± 4.52 | 32.18 ± 1.31 | 36.30 ± 1.71 |
| Quercetin-3-O-rhamnoside | 8.81 ± 0.50 | - | - |
| Valoneic acid dilactone | 13.127.81 ± 744.41 | 5947.83 ± 461.67 | 11.649.89 ± 559.83 |
| Phloretin | 39.84 ± 2.32 | 9.31 ± 0.43 | 10.38 ± 1.10 |
| Phloridzin | 9.21 ± 0.60 | 4.34 ± 0.19 | - |
| Myricitrin | 74.35 ± 3.26 | 25.49 ± 1.87 | 41.91 ± 2.90 |
| Sinensetin | 7.38 ± 0.60 | - | - |
| Rutin | 54.54 ± 5.28 | 17.61 ± 1.43 | 3.80 ± 0.19 |
| TAC | DPPH | TP | TF | TCT | AsA | TCHL | TCAR | |
|---|---|---|---|---|---|---|---|---|
| C | ||||||||
| Peel | 17 a | 42 | 4.2 a | 17 a | 10 b | 4.3 b | 0.23 a | 0.15 a |
| Pulp | 4.8 b | 14 | 0.9 c | 0.6 c | 4.0 c | 3.6 b | 0.05 c | 0.04 b |
| Seed | 17 a | 62 | 2.1 b | 6.3 b | 11 a | 12 a | 0.12 b | 0.02 c |
| RS | ||||||||
| UF | 10 c | 36 | 2.3 b | 8.0 a | 7.6 b | 4.9 c | 0.24 a | 0.04 c |
| SRF | 13 b | 38 | 1.9 c | 6.9 b | 6.5 c | 6.9 b | 0.06 b | 0.07 b |
| RF | 16 a | 43 | 3.0 a | 8.6 a | 12 a | 8.4 a | 0.09 b | 0.11 a |
| Interaction | ||||||||
| Peel × UF | 19 c | 47 d | 5.2 a | 18 a | 10 b | 1.6 f | 0.50 a | 0.06 c |
| Pulp × UF | 5.7 f | 9.8 h | 0.9 e | 0.5 e | 3.7 d | 4.2 e | 0.06 c | 0.02 d |
| Seed × UF | 5.8 f | 51 c | 0.8 e | 5.7 d | 8.9 b | 8.7 c | 0.17 b | 0.03 d |
| Peel × SRF | 16 e | 42 e | 3.9 c | 15 b | 5.7 c | 4.2 e | 0.10 b | 0.14 b |
| Pulp × SRF | 3.7 g | 11 h | 0.8 e | 0.5 e | 3.8 d | 4.3 e | 0.03 c | 0.05 c |
| Seed × SRF | 20 b | 63 b | 1.0 e | 5.1 d | 10 b | 12 b | 0.06 c | 0.01 d |
| Peel × RF | 17 d | 37 f | 3.5 d | 17 a | 15 a | 6.9 d | 0.10 b | 0.24 a |
| Pulp × RF | 5.1 f | 21 g | 0.9 e | 0.7 e | 4.6 d | 2.2 f | 0.04 c | 0.06 c |
| Seed × RF | 24 a | 72 a | 4.4 b | 8.0 c | 15 a | 16 a | 0.12 b | 0.02 d |
| Significance | ||||||||
| C | *** | *** | *** | *** | *** | *** | *** | *** |
| RS | *** | *** | *** | *** | *** | *** | *** | *** |
| C × RS | *** | *** | *** | *** | *** | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanzo, G.; Vitale, E.; Iesce, M.R.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening. Antioxidants 2022, 11, 187. https://doi.org/10.3390/antiox11020187
Costanzo G, Vitale E, Iesce MR, Naviglio D, Amoresano A, Fontanarosa C, Spinelli M, Ciaravolo M, Arena C. Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening. Antioxidants. 2022; 11(2):187. https://doi.org/10.3390/antiox11020187
Chicago/Turabian StyleCostanzo, Giulia, Ermenegilda Vitale, Maria Rosaria Iesce, Daniele Naviglio, Angela Amoresano, Carolina Fontanarosa, Michele Spinelli, Martina Ciaravolo, and Carmen Arena. 2022. "Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening" Antioxidants 11, no. 2: 187. https://doi.org/10.3390/antiox11020187
APA StyleCostanzo, G., Vitale, E., Iesce, M. R., Naviglio, D., Amoresano, A., Fontanarosa, C., Spinelli, M., Ciaravolo, M., & Arena, C. (2022). Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening. Antioxidants, 11(2), 187. https://doi.org/10.3390/antiox11020187