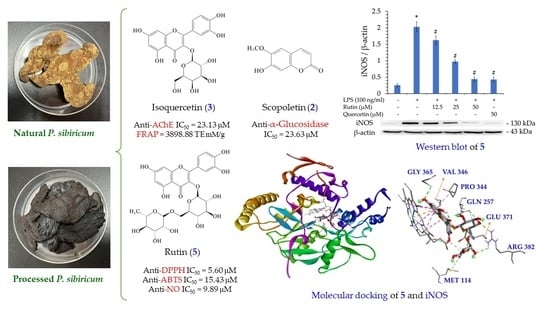
Main Bioactive Components and Their Biological Activities from Natural and Processed Rhizomes of Polygonum sibiricum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of P. sibiricum Extracts
2.3. Preparation of Bioactive Components
2.4. Reverse-Phase HPLC
2.5. Measurement of Total Phenolic Content (TPC)
2.6. Measurement of Total Flavonoid Content (TFC)
2.7. DPPH Radical Scavenging Assay
2.8. ABTS Radical Scavenging Assay
2.9. Superoxide Radical Scavenging Assay
2.10. Ferric Reducing Antioxidant Power (FRAP) Assay
2.11. α-Glucosidase Inhibitory Assay
2.12. Acetylcholinesterase Inhibitory Assay
2.13. Cell Culture
2.14. Nitric Oxide Inhibitory Assay
2.15. MTT Assay
2.16. Western Blot Analysis
2.17. Molecular Modeling Docking Study
2.18. Statistical Analysis
3. Results and Discussion
3.1. Measurement of TPC, TFC and Yield in Various Solvent Extracts
3.2. DPPH Free-Radical Scavenging Effect of Various Solvent Extracts
3.3. ABTS Free-Radical Scavenging Effect of Various Solvent Extracts
3.4. Superoxide Radical Scavenging Effect of Various Solvent Extracts
3.5. Ferric Reducing Antioxidant Power (FRAP) Effect of Various Solvent Extracts
3.6. Anti-α-Glucosidase Effect of Various Solvent Extracts
3.7. Acetylcholinesterase (AChE) Inhibitory Effect of Various Solvent Extracts
3.8. Nitric Oxide Inhibitory (NO) Effect of Various Solvent Extracts
3.9. MTT Assay of Various Solvent Extracts
3.10. Quantitation of Bioactive Components in Various Solvent Extracts
3.11. Antioxidant Effects of Isolated Components
3.12. Anti-α-Glucosidase Effects of Isolated Components
3.13. Acetylcholinesterase (AChE) Inhibitory Effects of Isolated Compounds
3.14. Nitric Oxide (NO) Inhibitory Effect of Isolated Components
3.15. MTT Assay of Isolated Components
3.16. Western Blot Analysis of Isolated Components
3.17. Molecular Docking Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, P.-Y.; Guo, Y.-J.; Tian, Y.-S.; Gu, L.-F.; Qi, J.; Yu, B.-Y. Reverse tracing anti-thrombotic active ingredients from dried Rehmannia Radix based on multidimensional spectrum-effect relationship analysis of steaming and drying for nine cycles. J. Ethnopharmacol. 2021, 276, 114177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hao, F.; Yao, Z.; Zhu, J.; Jing, X.; Wang, X. Efficient extraction of flavonoids from Polygonatum sibiricum using a deep eutectic solvent as a green extraction solvent. Microchem. J. 2022, 175, 107168. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.J.; Li, X.F.; Sun, Y.J.; Li, H.W.; Su, F.Y.; Feng, W.S. Homoisoflavanones with estrogenic activity from the rhizomes of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 2018, 20, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.R.; Li, X.; Wang, S.X. Two new alkaloids from the rhizome of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 2005, 7, 127–130. [Google Scholar] [CrossRef]
- Son, K.H.; Do, J.C.; Kang, S.S. Steroidal saponins from the rhizomes of Polygonatum sibiricum. J. Nat. Prod. 1990, 53, 333–339. [Google Scholar] [CrossRef]
- Hu, C.Y.; Xu, D.P.; Wu, Y.M.; Ou, S.Y. Triterpenoid saponins from the rhizome of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 2010, 12, 801–808. [Google Scholar] [CrossRef]
- Zheng, S. Protective effect of Polygonatum sibiricum Polysaccharide on D-galactose-induced aging rats model. Sci. Rep. 2020, 10, 2246. [Google Scholar] [CrossRef] [Green Version]
- QIN, Z. Effect of rhizoma polygonati on functional activity of endothelial progenitor cells to delay senescense via decrease of ros. Chin. Pharmacol. Bull. 2019, 12, 123–127. [Google Scholar]
- Debnath, T.; Park, S.R.; Jo, J.E.; Lim, B.O. Antioxidant and anti-inflammatory activity of Polygonatum sibiricum rhizome extracts. Asian Pacific J. Trop. Dis. 2013, 3, 308–313. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Q.-L.; Hou, S.-B.; Chen, G. Chemical constituents from the rhizomes of Polygonatum sibiricum Red. and anti-inflammatory activity in RAW264. 7 macrophage cells. Nat. Prod. Res. 2019, 33, 2359–2362. [Google Scholar] [CrossRef]
- Liu, J.; Li, T.; Chen, H.; Yu, Q.; Yan, C. Structural characterization and osteogenic activity in vitro of novel polysaccharides from the rhizome of Polygonatum sibiricum. Food Funct. 2021, 12, 6626–6636. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Nong, M.-N.; Zhao, J.-M.; Peng, X.-M.; Zong, S.-H.; Zeng, G.-F. Polygonatum sibiricum polysaccharide inhibits osteoporosis by promoting osteoblast formation and blocking osteoclastogenesis through Wnt/β-catenin signalling pathway. Sci. Rep. 2016, 6, 32261. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, L.; Jiang, P.; Xu, G.; Sun, T. Immunological regulation of the active fraction from Polygonatum sibiricum F. Delaroche based on improvement of intestinal microflora and activation of RAW264. 7 cells. J. Ethnopharmacol. 2022, 293, 115240. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Dong, Z.; Zhu, X.; Xu, H.; Zhao, Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107, 796–802. [Google Scholar] [CrossRef]
- Huang, S.; Yuan, H.; Li, W.; Liu, X.; Zhang, X.; Xiang, D.; Luo, S. Polygonatum sibiricum polysaccharides protect against MPP-induced neurotoxicity via the Akt/mTOR and Nrf2 pathways. Oxid. Med. Cell. Longev. 2021, 88, 889. [Google Scholar] [CrossRef]
- Yu, L.-Z.; Zhang, X.-P.; Wang, Y.-X. Polygonatum sibiricum extract exerts inhibitory effect on diabetes in a rat model. Trop. J. Pharm. Res. 2019, 18, 1493–1497. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, Z.; Yang, R.; Ye, Y.; Pei, L.; Xiong, S.; Wang, S.; Wang, L.; Liu, S. Polysaccharide-rich extract from Polygonatum sibiricum protects hematopoiesis in bone marrow suppressed by triple negative breast cancer. Biomed. Pharmacother. 2021, 137, 111338. [Google Scholar] [CrossRef]
- Jo, K.; Suh, H.J.; Choi, H.S. Polygonatum sibiricum rhizome promotes sleep by regulating non-rapid eye movement and GABAergic/serotonergic receptors in rodent models. Biomed. Pharmacother. 2018, 105, 167–175. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Hao, Y.; Du, K.; Du, H.; Ma, C.; Tu, H.; He, Y. A systematic review on botany, processing, application, phytochemistry and pharmacological action of Radix Rehmnniae. J. Ethnopharmacol. 2021, 285, 114820. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef]
- Droadowski, L.A.; Thomson, A.B. Intestinal sugar transport. World J. Gastroenterol. 2006, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.; Sousa, J.L.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, V.P.; Zhu, X.; Perry, G.; Smith, M.A. Oxidative stress in diabetes and Alzheimer’s disease. J. Alzheimers Dis. 2009, 16, 763–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.-H.; Lee, H.-K.; Kim, J.-A.; Hong, S.-I.; Kim, H.-C.; Jo, T.-H.; Park, Y.-I.; Lee, C.-K.; Kim, Y.-B.; Lee, S.-Y. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Van’T Hof, R.J.; Ralston, S.H. Nitric oxide and bone. Immunology 2001, 103, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- Liang, Y.-C.; Huang, Y.-T.; Tsai, S.-H.; Lin-Shiau, S.-Y.; Chen, C.-F.; Lin, J.-K. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis 1999, 20, 1945–1952. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.C.; Yang, C.S.; Cheng, M.J.; Fu, S.L.; Chen, J.J. Comparison of various solvent extracts and major bioactive components from unsalt-fried and salt-fried rhizomes of Anemarrhena asphodeloides for antioxidant, anti-α-glucosidase, and an-ti-acetylcholinesterase activities. Antioxidants 2022, 11, 385. [Google Scholar] [CrossRef]
- Lin, Y.T.; Lin, H.R.; Yang, C.S.; Liaw, C.C.; Sung, P.J.; Kuo, Y.H.; Cheng, M.J.; Chen, J.J. Antioxidant and Anti-α-glucosidase activities of various solvent extracts and major bioactive components from the fruits of Crataegus pinnatifida. Antioxidants 2022, 11, 320. [Google Scholar] [CrossRef]
- Noreen, H.; Semmar, N.; Farman, M.; McCullagh, J.S. Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus didymus. Asian Pac. J. Trop. Med. 2017, 10, 792–801. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.K.; Singh, A.P. In vitro antioxidant and free radical scavenging activity of Nardostachys jatamansi DC. J. Acupunct. Meridian Stud. 2012, 5, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-M.; Wang, M.-H.; Rhee, H.-I. A novel α-glucosidase inhibitor from pine bark. Carbohydr. Res. 2004, 339, 715–717. [Google Scholar] [CrossRef]
- Tran, T.-D.; Nguyen, T.-C.-V.; Nguyen, N.-S.; Nguyen, D.-M.; Nguyen, T.-T.-H.; Le, M.-T.; Thai, K.-M. Synthesis of novel chalcones as acetylcholinesterase inhibitors. Appl. Sci. 2016, 6, 198. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.J.; Lee, S.S.; Chen, S.C.; Ho, F.M.; Lin, W.W. Oregonin inhibits lipopolysaccharide-induced iNOS gene transcription and upregulates HO-1 expression in macrophages and microglia. Br. J. Pharmacol. 2005, 146, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- BIOVIA. Dassault Systèmes. In Discovery Studio Client 2021, v.21.1.0; Dassault Systèmes: San Diego, CA, USA, 2021. [Google Scholar]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Bourne, Y.; Grassi, J.; Bougis, P.E.; Marchot, P. Conformational flexibility of the acetylcholinesterase tetramer suggested by X-ray crystallography. J. Biol. Chem. 1999, 274, 30370–30376. [Google Scholar] [CrossRef] [Green Version]
- Francis, S.M.; Mittal, A.; Sharma, M.; Bharatam, P.V. Design of Benzene-1,2-diamines as selective inducible nitric oxide synthase inhibitors: A combined de novo design and docking analysis. J. Mol. Model. 2008, 14, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Mbeunkui, F.; Grace, M.H.; Lategan, C.; Smith, P.J.; Raskin, I.; Lila, M.A. Isolation and identification of antiplasmodial N-alkylamides from Spilanthes acmella flowers using centrifugal partition chromatography and ESI-ITTOF-MS. J. Chromatogr. B 2011, 879, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Mareček, V.; Mikyška, A.; Hampel, D.; Čejka, P.; Neuwirthová, J.; Malachová, A.; Cerkal, R. ABTS and DPPH methods as a tool for studying antioxidant capacity of spring barley and malt. J. Cereal Sci. 2017, 73, 40–45. [Google Scholar] [CrossRef]
- Raghavendra, R.; Neelagund, S.; Kuluvar, G.; Bhanuprakash, V.; Revanaiah, Y. Protective effect of partially purified 35 kDa protein from silk worm (Bombyx mori) fecal matter against carbon tetrachloride induced hepatotoxicity and in vitro anti-viral properties. Pharm. Biol. 2010, 12, 1426–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Zaheer, J.; Najam-Us-Saqib, Q.; Qamar, M.; Akram, M. In vitro (anti-alpha-glucosidase) activity and in vivo anti-diabetic activity of Androsace foliosa (common rock jasmine) in alloxan-induced diabetic BALB/c mice. Eur. J. Inflamm. 2019, 17, 2058739219857429. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Li, X.; Meng, S.; Ma, T.; Wan, L.; Xu, S. Chlorogenic acid alleviates Aβ25-35-induced autophagy and cognitive impairment via the mTOR/TFEB signaling pathway. Drug Des. Dev. Ther. 2020, 14, 1705. [Google Scholar] [CrossRef]
- Yang, Q.; Kang, Z.; Zhang, J.; Qu, F.; Song, B. Neuroprotective effects of isoquercetin: An in vitro and in vivo study. Cell J. 2021, 23, 355. [Google Scholar]













| Extracting Solvents | TPC (mg/g) a (GAE) | TFC (mg/g) b (QCE) | Yields (%) c | |||
|---|---|---|---|---|---|---|
| PS | PPS | PS | PPS | PS | PPS | |
| Dichloromethane | 77.50 ± 7.34 * | 52.52 ± 0.47 ** | 65.15 ± 6.51 *** | 26.86 ± 5.81 ** | 0.35 ± 0.12 | 1.25 ± 0.03 |
| Ethyl acetate | 54.84 ± 4.56 * | 60.47 ± 1.91 * | 86.02 ± 1.54 *** | 98.30 ± 0.47 *** | 1.23 ± 0.50 | 2.25 ± 0.01 |
| Acetone | 75.61 ± 7.51 * | 53.11 ± 2.26 ** | 25.21 ± 5.84 *** | 20.70 ± 4.56 ** | 0.54 ± 0.06 | 3.75 ± 0.15 |
| Ethanol | 50.82 ± 7.56 * | 26.52 ± 1.62 * | 14.51 ± 3.27 * | 19.66 ± 4.38 ** | 5.32 ± 0.58 | 5.60 ± 0.89 |
| Methanol | 46.37 ± 5.44 * | 28.92 ± 2.46 ** | 9.94 ± 1.34 *** | 22.02 ± 4.44 ** | 13.49 ± 1.53 | 10.45 ± 1.57 |
| Water | 36.87 ± 3.86 ** | 32.55 ± 2.34 ** | 12.22 ± 3.47 *** | 21.13 ± 2.51 *** | 5.36 ± 0.97 | 15.90 ± 2.64 |
| Extracting Solvents | SC50 (μg/mL) a | TE (mM/g) c | ||||||
|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | Superoxide | FRAP | |||||
| PS | PPS | PS | PPS | PS | PPS | PS | PPS | |
| Dichloromethane | 236.14 ± 3.89 *** | >400 | 240.49 ± 5.68 *** | >400 | >400 | >400 | 667.08 ± 18.56 ** | 322.26 ± 5.46 * |
| Ethyl acetate | >400 | >400 | >400 | >400 | 190.23 ± 1.09 *** | >400 | 651.03 ± 20.56 ** | 604.38 ± 3.73 * |
| Acetone | 278.31 ± 3.26 *** | >400 | 229.37 ± 5.59 *** | >400 | >400 | >400 | 515.84 ± 24.86 ** | 362.87 ± 7.48 * |
| Ethanol | >400 | >400 | 245.48 ± 3.59 *** | >400 | >400 | >400 | 431.13 ± 18.70 ** | 234.39 ± 1.75 ** |
| Methanol | >400 | >400 | 346.48 ± 3.21 *** | >400 | >400 | >400 | 414.40 ± 17.74 ** | 203.03 ± 4.08 * |
| Water | >400 | >400 | >400 | >400 | 294.54 ± 7.28 ** | >400 | 296.71 ± 16.46 * | 116.01 ± 2.11 * |
| BHT b | 35.54 ± 0.64 ** | 20.57 ± 0.22 ** | N.A. d | 3005.93 ± 13.10 *** | ||||
| Extracting Solvents | α-Glucosidase IC50 (μg/mL) a | AChE IC50 (μg/mL) a | ||
|---|---|---|---|---|
| PS | PPS | PS | PPS | |
| Dichloromethane | 34.29 ± 6.26 * | >600 | 60.90 ± 6.18 ** | 56.27 ± 7.11 *** |
| Ethyl acetate | 22.34 ± 1.66 ** | >600 | 86.91 ± 3.25 ** | 32.60 ± 5.27 * |
| Acetone | 26.13 ± 2.48 *** | >600 | 65.54 ± 10.70 ** | 68.45 ± 4.95 *** |
| Ethanol | >600 | >600 | 63.41 ± 7.58 ** | >400 |
| Methanol | >600 | >600 | 65.59 ± 5.83 ** | >400 |
| Water | >600 | >600 | 94.07 ± 5.00 | >400 |
| Acarbose b | 379.07 ± 4.23 * | — | ||
| Chlorogenic acid b | — | 23.27 ± 0.10 * | ||
| Extracting Solvents | Nitric Oxide IC50 (μg/mL) a | |
|---|---|---|
| PS | PPS | |
| Dichloromethane | 18.84 ± 1.80 ** | 27.48 ± 6.99 ** |
| Ethyl acetate | 45.22 ± 6.80 ** | 61.08 ± 2.88 ** |
| Acetone | 40.68 ± 6.13 ** | 105.94 ± 8.63 * |
| Ethanol | 81.23 ± 2.26 * | 181.80 ± 7.63 * |
| Methanol | 91.14 ± 8.18 * | 157.43 ± 9.56 * |
| Water | 176.82 ± 8.64 * | 202.85 ± 19.41 * |
| Quercetin b | 7.52 ± 0.25 * | |
| Extracting Solvents | 5-HMF (mg/g) | Scopoletin (mg/g) | Rutin (mg/g) | Hyperoside (mg/g) | Isoquercetin (mg/g) | Total Amount (mg/g) |
|---|---|---|---|---|---|---|
| Water (PS) | 3.73 ± 0.22 | N.D. a | 1.80 ± 0.18 | N.D. a | 1.24 ± 0.10 | 6.77 ± 0.50 |
| Methanol (PS) | 5.73 ± 0.36 | 1.17 ± 0.08 | 1.60 ± 0.11 | 2.14 ± 0.22 | 2.66 ± 0.13 | 13.30 ± 0.90 |
| Ethanol (PS) | 6.43 ± 0.48 | 2.85 ± 0.19 | 1.51 ± 0.10 | 1.44 ± 0.09 | 1.26 ± 0.13 | 13.49 ± 0.99 |
| Acetone (PS) | 5.23 ± 0.61 | 2.33 ± 0.22 | 1.24 ± 0.09 | 1.21 ± 0.16 | 1.02 ± 0.06 | 11.03 ± 1.14 |
| Ethyl acetate (PS) | 3.84 ± 0.22 | 4.69 ± 0.35 | 1.32 ± 0.07 | 2.34 ± 0.28 | 1.22 ± 0.08 | 13.41 ± 1.00 |
| Dichloromethane (PS) | 5.63 ± 0.48 | 2.12 ± 0.34 | 3.21 ± 0.33 | 1.93 ± 0.09 | 4.36 ± 0.44 | 17.25 ± 1.68 |
| Water (PPS) | 12.81 ± 2.38 | N.D. a | 1.30 ± 0.07 | 1.24 ± 0.09 | 1.84 ± 0.12 | 17.19 ± 2.66 |
| Methanol (PPS) | 20.83 ± 1.84 | N.D. a | 1.82 ± 0.11 | 1.24 ± 0.13 | 1.74 ± 0.18 | 25.63 ± 2.26 |
| Ethanol (PPS) | 22.43 ± 2.12 | N.D. a | N.D. a | 3.64 ± 0.32 | N.D. a | 26.07 ± 2.44 |
| Acetone (PPS) | 24.63 ± 1.92 | N.D. a | N.D. a | 2.26 ± 0.13 | N.D. a | 26.89 ± 2.05 |
| Ethyl acetate (PPS) | 21.86 ± 2.13 | N.D. a | N.D. a | 3.44 ± 0.31 | N.D. a | 25.03 ± 2.44 |
| Dichloromethane (PPS) | 23.86 ± 1.88 | N.D. a | N.D. a | 3.63 ± 0.43 | N.D. a | 27.49 ± 2.31 |
| Compounds | SC50 (μM) a | (mM/g) (TE) c | ||
|---|---|---|---|---|
| DPPH | ABTS | Superoxide | FRAP | |
| 5-HMF (1) | >400 | >400 | >400 | 26.70 ± 0.97 * |
| Scopoletin (2) | >400 | 91.27 ± 3.36 * | >400 | 2892.97 ± 19.18 *** |
| Isoquercetin (3) | 12.64 ± 3.21 * | 22.73 ± 1.17 * | 179.62 ± 4.43 ** | 3898.88 ± 23.23 *** |
| Hyperoside (4) | 12.46 ± 4.02 * | 29.26 ± 0.51 * | 172.50 ± 3.80 ** | 3246.93 ± 31.92 *** |
| Rutin (5) | 5.60 ± 0.34 *** | 15.43 ± 0.25 ** | 174.82 ± 3.02 ** | 2221.33 ± 5.02 *** |
| BHT b | 192.28 ± 8.94 * | 100.35 ± 7.26 * | N.A. d | 2896.93 ± 21.19 *** |
| Compounds | α-Glucosidase | AChE |
|---|---|---|
| IC50 (μM) a | ||
| 5-HMF (1) | >600 | 81.46 ± 11.05 ** |
| Scopoletin (2) | 23.63 ± 7.22 *** | 32.35 ± 2.05 ** |
| Isoquercetin (3) | 159.73 ± 3.12 *** | 23.13 ± 3.15 *** |
| Hyperoside (4) | 208.14 ± 5.70 *** | 121.10 ± 10.70 ** |
| Rutin (5) | 331.15 ± 3.81 ** | 33.09 ± 5.43 ** |
| Acarbose b | 550.15 ± 7.65 * | — |
| Chlorogenic acid b | — | 68.23 ± 2.90 * |
| Compounds | Nitric Oxide |
|---|---|
| IC50 (μM) a | |
| 5-HMF (1) | 34.90 ± 8.80 * |
| Scopoletin (2) | 36.26 ± 4.65 * |
| Isoquercetin (3) | 17.03 ± 1.28 ** |
| Hyperoside (4) | 18.87 ± 1.68 * |
| Rutin (5) | 9.89 ± 1.36 ** |
| Quercetin b | 18.26 ± 0.54 * |
| Compounds | Affinity (kcal/mol) |
|---|---|
| 5-HMF (1) | −4.8 |
| Scopoletin (2) | −7.0 |
| Isoquercetin (3) | −7.5 |
| Hyperoside (4) | −3.2 |
| Rutin (5) | −6.8 |
| Chlorogenic acid a | −5.3 |
| Compounds | Affinity (kcal/mol) |
|---|---|
| 5-HMF (1) | −5.8 |
| Scopoletin (2) | −5.5 |
| Isoquercetin (3) | −7.3 |
| Hyperoside (4) | −6.8 |
| Rutin (5) | −9.5 |
| Quercetin a | −7.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-C.; Yang, C.-S.; Chen, J.-J. Main Bioactive Components and Their Biological Activities from Natural and Processed Rhizomes of Polygonum sibiricum. Antioxidants 2022, 11, 1383. https://doi.org/10.3390/antiox11071383
Chen S-C, Yang C-S, Chen J-J. Main Bioactive Components and Their Biological Activities from Natural and Processed Rhizomes of Polygonum sibiricum. Antioxidants. 2022; 11(7):1383. https://doi.org/10.3390/antiox11071383
Chicago/Turabian StyleChen, Shih-Chi, Chang-Syun Yang, and Jih-Jung Chen. 2022. "Main Bioactive Components and Their Biological Activities from Natural and Processed Rhizomes of Polygonum sibiricum" Antioxidants 11, no. 7: 1383. https://doi.org/10.3390/antiox11071383
APA StyleChen, S. -C., Yang, C. -S., & Chen, J. -J. (2022). Main Bioactive Components and Their Biological Activities from Natural and Processed Rhizomes of Polygonum sibiricum. Antioxidants, 11(7), 1383. https://doi.org/10.3390/antiox11071383