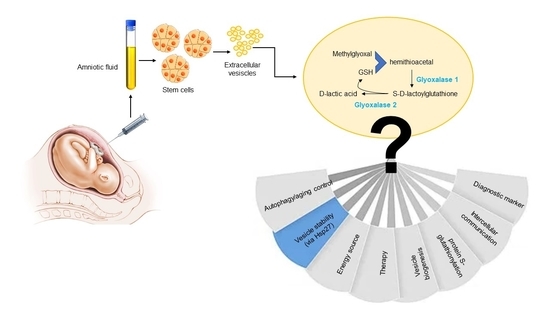
The Glyoxalase System Is a Novel Cargo of Amniotic Fluid Stem-Cell-Derived Extracellular Vesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HASC Isolation and Culture
2.3. HASC-Derived EV Isolation and Culture
2.4. HASC-Derived EV Characterization
2.5. Western Blot Analysis
2.6. Glyoxalase-Specific Activity
2.7. MG-H1 Detection
2.8. Immunoprecipitation
2.9. Glutathione (GSH) Assay
2.10. D-Lactate Assay
2.11. Statistical Analysis
3. Results
3.1. Glyoxalases Are Cargos of Both HASC-P10 and HASC-P100 EVs
3.2. Glyoxalases Are Functional in Both HASC-P10 and HASC-P100 EVs
3.3. HASC-P10 and HASC-P100 EVs Contain MG-H1
3.4. The Receptor for Advanced Glycation End Products (RAGE) Is not Present in HASC-P10 and HASC-P100 EVs
3.5. Heat Shock Protein (Hsp)27 Is a MG-H1-Modified Protein in HASC-P10 and HASC-P100 EVs
3.6. HASC-P10 and HASC-P100 EVs Contain GSH and D-lactate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antognelli, C.; Talesa, V.N. Glyoxalases in Urological Malignancies. Int. J. Mol. Sci. 2018, 19, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornalley, P.J. The glyoxalase system: New developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem. J. 1990, 269, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Talesa, V.N.; Ferri, I.; Bellezza, G.; Love, H.D.; Sidoni, A.; Antognelli, C. Glyoxalase 2 Is Involved in Human Prostate Cancer Progression as Part of a Mechanism Driven By PTEN/PI3K/AKT/mTOR Signaling with Involvement of PKM2 and ERα. Prostate 2017, 77, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Sousa Silva, M.; Gomes, R.A.; Ferreira, A.E.; Ponces Freire, A.; Cordeiro, C. The glyoxalase pathway: The first hundred years and beyond. Biochem. J. 2013, 453, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Antognelli, C.; Ferri, I.; Bellezza, G.; Siccu, P.; Love, H.D.; Talesa, V.N.; Sidoni, A. Glyoxalase 2 drives tumorigenesis in human prostate cells in a mechanism involving androgen receptor and p53-p21 axis. Mol. Carcinog. 2017, 56, 2112–2126. [Google Scholar] [CrossRef]
- Antognelli, C.; Frosini, R.; Santolla, M.F.; Peirce, M.J.; Talesa, V.N. Oleuropein-Induced Apoptosis Is Mediated by Mitochondrial Glyoxalase 2 in NSCLC A549 Cells: A Mechanistic Inside and a Possible Novel Nonenzymatic Role for an Ancient Enzyme. Oxidative Med. Cell. Longev. 2019, 22, 8576961. [Google Scholar] [CrossRef] [Green Version]
- De Bari, L.; Scirè, A.; Minnelli, C.; Cianfruglia, L.; Kalapos, M.P.; Armeni, T. Interplay among Oxidative Stress, Methylglyoxal Pathway and S-Glutathionylation. Antioxidants 2020, 10, 19. [Google Scholar] [CrossRef]
- Donnellan, L.; Young, C.; Simpson, B.S.; Acland, M.; Dhillon, V.S.; Costabile, M.; Fenech, M.; Hoffmann, P.; Deo, P. Proteomic Analysis of Methylglyoxal Modifications Reveals Susceptibility of Glycolytic Enzymes to Dicarbonyl Stress. Int. J. Mol. Sci. 2022, 23, 3689. [Google Scholar] [CrossRef]
- Nass, N.; Vogel, K.; Hofmann, B.; Presek, P.; Silber, R.E.; Simm, A. Glycation of PDGF results in decreased biological activity. Int. J. Biochem. Cell Biol. 2010, 42, 749–754. [Google Scholar] [CrossRef]
- Bansode, S.B.; Chougale, A.D.; Joshi, R.S.; Giri, A.P.; Bodhankar, S.L.; Harsulkar, A.M.; Kulkarni, M.J. Proteomic analysis of protease resistant proteins in the diabetic rat kidney. Mol. Cell. Proteom. 2013, 12, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Dicarbonyl stress, protein glycation and the unfolded protein response. Glycoconj. J. 2021, 38, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Mashima, T.; Yamamoto, K.; Tsuruo, T. Modulation of heat-shock protein 27 (Hsp27) anti-apoptotic activity by methylglyoxal modification. J. Biol. Chem. 2002, 277, 45770–45775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schalkwijk, C.G.; van Bezu, J.; van der Schors, R.C.; Uchida, K.; Stehouwer, C.D.A.; van Hinsbergh, V.W.M. Heat-shock protein 27 is a major methylglyoxal-modified protein in endothelial cells. FEBS Lett. 2006, 580, 1565. [Google Scholar] [CrossRef] [Green Version]
- Sreejayan, N.; Yang, X.; Palanichamy, K.; Dolence, K.; Ren, J. Antioxidant properties of argpyrimidine. Eur. J. Pharmacol. 2008, 593, 30–35. [Google Scholar] [CrossRef]
- Brown, B.E.; Nobecourt, E.; Zeng, J.; Jenkins, A.J.; Rye, K.A.; Davies, M.J. Apolipoprotein A-I glycation by glucose and reactive aldehydes alters phospholipid affinity but not cholesterol export from lipid-laden macrophages. PLoS ONE 2013, 8, e65430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Castillo, C.; Shuck, S.C. Diet and Obesity-Induced Methylglyoxal Production and Links to Metabolic Disease. Chem. Res. Toxicol. 2021, 34, 2424–2440. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.E.; Rashid, I.; van Reyk, D.M.; Davies, M.J. Glycation of low-density lipoprotein results in the time-dependent accumulation of cholesteryl esters and apolipoprotein B-100 protein in primary human monocyte-derived macrophages. FEBS J. 2007, 274, 1530–1541. [Google Scholar] [CrossRef]
- Tatone, C.; Heizenrieder, T.; Di Emidio, G.; Treffon, P.; Amicarelli, F.; Seidel, T.; Eichenlaub-Ritter, U. Evidence that carbonyl stress by methylglyoxal exposure induces DNA damage and spindle aberrations, affects mitochondrial integrity in mammalian oocytes and contributes to oocyte ageing. Hum. Reprod. 2011, 26, 1843–1859. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Mun, D.; Kang, J.Y.; Lee, S.H.; Yun, N.; Joung, B. Improved cardiac-specific delivery of RAGE siRNA within small extracellular vesicles engineered to express intense cardiac targeting peptide attenuates myocarditis. Mol. Ther. Nucleic Acids 2021, 24, 1024–1032. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Extracellular Vesicles and Thrombogenicity in Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 1774. [Google Scholar] [CrossRef]
- Haddad, M.; Perrotte, M.; Khedher, M.R.B.; Demongin, C.; Lepage, A.; Fülöp, T.; Ramassamy, C. Methylglyoxal and Glyoxal as Potential Peripheral Markers for MCI Diagnosis and Their Effects on the Expression of Neurotrophic, Inflammatory and Neurodegenerative Factors in Neurons and in Neuronal Derived-Extracellular Vesicles. Int. J. Mol. Sci. 2019, 20, 4906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsakaneli, A.; Carregari, V.C.; Morini, M.; Eva, A.; Cangemi, G.; Chayka, O.; Makarov, E.; Bibbò, S.; Capone, E.; Sala, G.; et al. MYC regulates metabolism through vesicular transfer of glycolytic kinases. Open Biol. 2021, 11, 210276. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, K.G.; Ek, B.; Stavreus-Evers, A.; Larsson, A.; Ronquist, G. Human prostasomes express glycolytic enzymes with capacity for ATP production. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E576–E582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Göran Ronquist, K. Extracellular vesicles and energy metabolism. Clin. Chim. Acta 2019, 488, 116–121. [Google Scholar] [CrossRef]
- Iraci, N.; Gaude, E.; Leonardi, T.; Costa, A.S.H.; Cossetti, C.; Peruzzotti-Jametti, L.; Bernstock, J.D.; Saini, H.K.; Gelati, M.; Vescovi, A.L.; et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat. Chem. Biol. 2017, 13, 951–955. [Google Scholar] [CrossRef] [Green Version]
- Romani, R.; Pirisinu, I.; Calvitti, M.; Pallotta, M.T.; Gargaro, M.; Bistoni, G.; Vacca, C.; Di Michele, A.; Orabona, C.; Rosati, J.; et al. Stem cells from human amniotic fluid exert immunoregulatory function via secreted indoleamine 2,3-dioxygenase1. J. Cell. Mol. Med. 2015, 19, 1593–1605. [Google Scholar] [CrossRef]
- Mezzasoma, L.; Bellezza, I.; Orvietani, P.; Manni, G.; Gargaro, M.; Sagini, K.; Llorente, A.; Scarpelli, P.; Pascucci, L.; Cellini, B.; et al. Amniotic fluid stem cell-derived extracellular vesicles are independent metabolic units capable of modulating inflammasome activation in THP-1 cells. FASEB J. 2022, 36, e22218. [Google Scholar] [CrossRef]
- Romani, R.; Fallarino, F.; Pirisinu, I.; Calvitti, M.; Caselli, A.; Fiaschi, T.; Gamberi, T.; Matino, D.; Talesa, V.N.; Donti, E.; et al. Comparative proteomic analysis of two distinct stem-cell populations from human amniotic fluid. Mol. Biosyst. 2015, 11, 1622–1632. [Google Scholar] [CrossRef]
- Pariano, M.; Costantini, C.; Santarelli, I.; Puccetti, M.; Giovagnoli, S.; Talesa, V.N.; Romani, L.; Antognelli, C. Defective Glyoxalase 1 Contributes to Pathogenic Inflammation in Cystic Fibrosis. Vaccines 2021, 9, 1311. [Google Scholar] [CrossRef]
- Antognelli, C.; Gambelunghe, A.; Del Buono, C.; Murgia, N.; Talesa, V.N.; Muzi, G. Crystalline silica Min-U-Sil 5 induces oxidative stress in human bronchial epithelial cells BEAS-2B by reducing the efficiency of antiglycation and antioxidant enzymatic defenses. Chem. Biol. Interact. 2009, 182, 13–21. [Google Scholar] [CrossRef]
- Delle Monache, S.; Pulcini, F.; Frosini, R.; Mattei, V.; Talesa, V.N.; Antognelli, C. Methylglyoxal-Dependent Glycative Stress Is Prevented by the Natural Antioxidant Oleuropein in Human Dental Pulp Stem Cells through Nrf2/Glo1 Pathway. Antioxidants 2021, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Cianfruglia, L.; Perrelli, A.; Fornelli, C.; Magini, A.; Gorbi, S.; Salzano, A.M.; Antognelli, C.; Retta, F.; Benedetti, V.; Cassoni, P.; et al. KRIT1 Loss-Of-Function Associated with Cerebral Cavernous Malformation Disease Leads to Enhanced S-Glutathionylation of Distinct Structural and Regulatory Proteins. Antioxidants 2019, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tisi, A.; Pulcini, F.; Carozza, G.; Mattei, V.; Flati, V.; Passacantando, M.; Antognelli, C.; Maccarone, R.; Delle Monache, S. Antioxidant Properties of Cerium Oxide Nanoparticles Prevent Retinal Neovascular Alterations In Vitro and In Vivo. Antioxidants 2022, 11, 1133. [Google Scholar] [CrossRef]
- Antognelli, C.; Marinucci, L.; Frosini, R.; Macchioni, L.; Talesa, V.N. Metastatic Prostate Cancer Cells Secrete Methylglyoxal-Derived MG-H1 to Reprogram Human Osteoblasts into a Dedifferentiated, Malignant-like Phenotype: A Possible Novel Player in Prostate Cancer Bone Metastases. Int. J. Mol. Sci. 2021, 22, 10191. [Google Scholar] [CrossRef]
- Sudnitsyna, M.V.; Gusev, N.B. Methylglyoxal and Small Heat Shock Proteins. Biochemistry 2017, 82, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Sudnitsyna, M.V.; Gusev, N.B. Is the small heat shock protein HspB1 (Hsp27) a real and predominant target of methylglyoxal modification? Cell Stress Chaperones 2019, 24, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, J.; Campos Campos, M.; Nawroth, P.; Fleming, T. The Glyoxalase System-New Insights into an Ancient Metabolism. Antioxidant 2020, 9, 939. [Google Scholar] [CrossRef]
- Haddad, M.; Perrotte, M.; Ben Khedher, M.R.; Madec, E.; Lepage, A.; Fülöp, T.; Ramassamy, C. Levels of Receptor for Advanced Glycation End Products and Glyoxalase-1 in the Total Circulating Extracellular Vesicles from Mild Cognitive Impairment and Different Stages of Alzheimer’s Disease Patients. J. Alzheimer’s Dis. 2021, 84, 227–237. [Google Scholar] [CrossRef]
- Nokin, M.J.; Durieux, F.; Bellier, J.; Peulen, O.; Uchida, K.; Spiegel, D.A.; Cochrane, J.R.; Hutton, C.A.; Castronovo, V.; Bellahcène, A. Hormetic potential of methylglyoxal, a side-product of glycolysis, in switching tumours from growth to death. Sci. Rep. 2017, 7, 11722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorova, L.D.; Kovalchuk, S.I.; Popkov, V.A.; Chernikov, V.P.; Zharikova, A.A.; Khutornenko, A.A.; Zorov, S.D.; Plokhikh, K.S.; Zinovkin, R.A.; Evtushenko, E.A.; et al. Do Extracellular Vesicles Derived from Mesenchymal Stem Cells Contain Functional Mitochondria? Int. J. Mol. Sci. 2022, 23, 7408. [Google Scholar] [CrossRef] [PubMed]
- Rogalla, T.; Ehrnsperger, M.; Preville, X.; Kotlyarov, A.; Lutsch, G.; Ducasse, C.; Paul, C.; Wieske, M.; Arrigo, A.P.; Buchner, J.; et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J. Biol. Chem. 1999, 274, 18947–18956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, V.S.; Madala, S.K.; Trinath, J.; Reddy, G.B. Extracellular small heat shock proteins: Exosomal biogenesis and function. Cell Stress Chaperones 2018, 23, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.A.; Deep, G.; Brinkley, T.E. Detection of the receptor for advanced glycation endproducts in neuronally-derived exosomes in plasma. Biochem. Biophys. Res. Commun. 2018, 500, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Creighton, D.J.; Migliorini, M.; Pourmotabbed, T.; Guha, M.K. Optimization of efficiency in the glyoxalase pathway. Biochemistry 1988, 27, 7376–7384. [Google Scholar] [CrossRef]
- Ercolani, L.; Scirè, A.; Galeazzi, R.; Massaccesi, L.; Cianfruglia, L.; Amici, A.; Piva, F.; Urbanelli, L.; Emiliani, C.; Principato, G.; et al. A possible S-glutathionylation of specific proteins by glyoxalase II: An in vitro and in silico study. Cell Biochem. Funct. 2016, 34, 620–627. [Google Scholar] [CrossRef]
- Costa, A.; Quarto, R.; Bollini, S. Small Extracellular Vesicles from Human Amniotic Fluid Samples as Promising Theranostics. Int. J. Mol. Sci. 2022, 23, 590. [Google Scholar] [CrossRef]
- Jahangiri, B.; Saei, A.K.; Obi, P.O.; Asghari, N.; Lorzadeh, S.; Hekmatirad, S.; Rahmati, M.; Velayatipour, F.; Asghari, M.H.; Saleem, A.; et al. Exosomes, autophagy and ER stress pathways in human diseases: Cross-regulation and therapeutic approaches. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166484. [Google Scholar] [CrossRef]
- Langeh, U.; Kumar, V.; Kumar, A.; Kumar, P.; Singh, C.; Singh, A. Cellular and mitochondrial quality control mechanisms in maintaining homeostasis in ageing. Rejuvenation Res. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Fleming, T.H.; Theilen, T.M.; Masania, J.; Wunderle, M.; Karimi, J.; Vittas, S.; Bernauer, R.; Bierhaus, A.; Rabbani, N.; Thornalley, P.J.; et al. Aging-dependent reduction in glyoxalase 1 delays wound healing. Gerontology 2013, 59, 427–437. [Google Scholar] [CrossRef]
- Varderidou-Minasian, S.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: Challenges and opportunities. Theranostics 2020, 10, 5979–5997. [Google Scholar] [CrossRef]
- Radnaa, E.; Richardson, L.S.; Sheller-Miller, S.; Baljinnyam, T.; de Castro Silva, M.; Kumar Kammala, A.; Urrabaz-Garza, R.; Kechichian, T.; Kim, S.; Han, A.; et al. Extracellular vesicle mediated feto-maternal HMGB1 signaling induces preterm birth. Lab Chip 2021, 21, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Sheller-Miller, S.; Menon, R. Isolation and characterization of human amniotic fluid-derived exosomes. Methods Enzym. 2020, 645, 181–194. [Google Scholar] [CrossRef]
- Ebert, B.; Rai, A.J. Isolation and Characterization of Amniotic Fluid-Derived Extracellular Vesicles for Biomarker Discovery. Methods Mol. Biol. 2018, 1885, 287–294. [Google Scholar]
- Bouvier, D.; Giguère, Y.; Blanchon, L.; Bujold, E.; Pereira, B.; Bernard, N.; Gallot, D.; Sapin, V.; Forest, J.C. Study of sRAGE, HMGB1, AGE, and S100A8/A9 Concentrations in Plasma and in Serum-Extracted Extracellular Vesicles of Pregnant Women with Preterm Premature Rupture of Membranes. Front. Physiol. 2020, 11, 609. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romani, R.; Talesa, V.N.; Antognelli, C. The Glyoxalase System Is a Novel Cargo of Amniotic Fluid Stem-Cell-Derived Extracellular Vesicles. Antioxidants 2022, 11, 1524. https://doi.org/10.3390/antiox11081524
Romani R, Talesa VN, Antognelli C. The Glyoxalase System Is a Novel Cargo of Amniotic Fluid Stem-Cell-Derived Extracellular Vesicles. Antioxidants. 2022; 11(8):1524. https://doi.org/10.3390/antiox11081524
Chicago/Turabian StyleRomani, Rita, Vincenzo Nicola Talesa, and Cinzia Antognelli. 2022. "The Glyoxalase System Is a Novel Cargo of Amniotic Fluid Stem-Cell-Derived Extracellular Vesicles" Antioxidants 11, no. 8: 1524. https://doi.org/10.3390/antiox11081524
APA StyleRomani, R., Talesa, V. N., & Antognelli, C. (2022). The Glyoxalase System Is a Novel Cargo of Amniotic Fluid Stem-Cell-Derived Extracellular Vesicles. Antioxidants, 11(8), 1524. https://doi.org/10.3390/antiox11081524