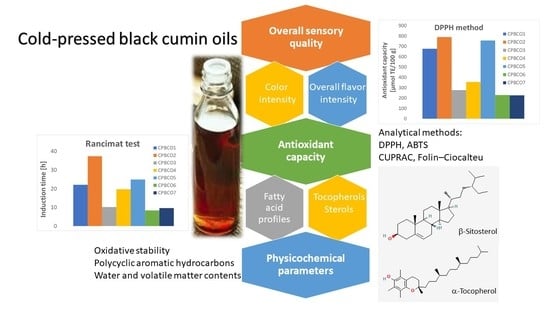
Effects of the Chemical Composition on the Antioxidant and Sensory Characteristics and Oxidative Stability of Cold-Pressed Black Cumin Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Determination of Tocopherols and Sterols Contents
2.4. Determination of Antioxidant Capacity
2.5. Determination of Oxidative Stability
2.6. Determination of Water and Volatile Matter Content
2.7. Determination of Polycyclic Aromatic Hydrocarbons
2.8. Determination of Fatty Acid Composition
2.9. Sensory Evaluation and Quality Assessment
2.10. Statistical Analysis
3. Results and Discussion
3.1. Tocopherol and Phytosterol Contents in Cold-Pressed Black Cumin Oils
3.2. Antioxidant Capacity of Cold-Pressed Black Cumin Oils
3.3. Oxidative Stability and Quality of Cold-Pressed Black Cumin Oils
3.3.1. Oxidative Stability
3.3.2. Amounts of Primary Oxidation Products and Free Fatty Acids
3.3.3. Water and Volatile Matter Contents
3.3.4. Polycyclic Aromatic Hydrocarbons Content
3.4. Fatty Acid Compositions of Cold-Pressed Black Cumin Oils
3.5. Sensory Analysis of Cold-Pressed Black Cumin Oils
3.6. Principal Component Analysis on the Entire Set of Chemical and Sensory Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AC | antioxidant capacity |
| AV | acid value |
| ANOVA | analysis of variance |
| AOCS | American Oil Chemists’ Society |
| B(a)A | benzo(a)anthracene |
| B(a)P | benzo(a)pyrene |
| B(b)F | benzo(b)fluoranthene |
| Chry | chrysene |
| CPBCO | cold-pressed black cumin oil |
| CUPRAC | Cupric-reducing antioxidant capacity |
| DGF | German Society for Fat Science |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EU | European Union |
| FAC | fatty acid composition |
| FAME | fatty acid methyl esters |
| FC | Folin–Ciocalteu |
| FFA | free fatty acids |
| FID | flame ionization detector |
| FRAP | Ferric-reducing antioxidant power |
| GA | gallic acid |
| GC-FID | gas chromatograph equipped with flame ionization detector |
| HAT | hydrogen atom transfer |
| HPLC | high performance liquid chromatography |
| HPLC-FLD | high performance liquid chromatography with the fluorescence detector |
| IC50 | half-maximal inhibitory concentration |
| IP | induction period |
| ISO | International Organization for Standardization |
| LDL | low-density lipoprotein |
| LOD | limit of detection |
| LOQ | limit of quantification |
| MUFA | monounsaturated fatty acids |
| OFI | overall flavor intensity |
| OSQ | overall sensory quality |
| PAH | polycyclic aromatic hydrocarbon |
| Σ4PAHs | sum of four specific polycyclic aromatic hydrocarbons |
| PC | principal component |
| PCA | principal component analysis |
| PUFA | polyunsaturated fatty acids |
| PV | peroxide value |
| QDA | quantitative descriptive analysis |
| SAFA | saturated fatty acids |
| SET | single electron transfer |
| SD | standard deviation |
| TE | Trolox equivalents |
| TPC | total phenolic content |
| TTC | total tocopherol content |
| TSC | total sterol content |
| WVC | water and volatile matter content |
References
- Dedebas, T.; Ekici, L.; Sagdic, O. Chemical characteristics and storage stabilities of different cold-pressed seed oils. J. Food Process. Preserv. 2021, 45, e15107. [Google Scholar] [CrossRef]
- Ketenoglu, O.; Kiralan, S.S.; Kiralan, M.; Ozkan, G.; Ramadan, M.F. Cold Pressed Black Cumin (Nigella sativa L.) Seed Oil. In Cold Pressed Oils; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2020; pp. 53–64. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Nigella (Black Cumin) Seed Oil. In Gourmet and Health-Promoting Specialty Oils, 3rd ed.; Moreau, R.A., Kamal-Eldin, A., Eds.; AOCS Press: Urbana, IL, USA; Elsevier: Amsterdam, The Netherlands, 2009; pp. 299–311. [Google Scholar] [CrossRef]
- Kabir, Y.; Shirakawa, H.; Komai, M. Nutritional composition of indigenous cultivar of black cumin seeds from Bangladesh. Prog. Nutr. 2019, 21, 428–434. [Google Scholar] [CrossRef]
- Cheikh-Rouhou, S.; Besbes, S.; Hentati, B.; Blecker, C.; Deroanne, C.; Attia, H. Nigella sativa L.: Chemical composition and physicochemical characteristics of lipid fraction. Food Chem. 2007, 101, 673–681. [Google Scholar] [CrossRef]
- Ramadan, M.F. Healthy blends of high linoleic sunflower oil with selected cold pressed oils: Functionality, stability and antioxidative characteristics. Ind. Crops Prod. 2013, 43, 65–72. [Google Scholar] [CrossRef]
- Tulukcu, E. A comparative study on fatty acid composition of black cumin obtained from different regions of Turkey, Iran and Syria. Afr. J. Agric. Res. 2011, 6, 892–895. [Google Scholar]
- Ramadan, M.F.; Mörsel, J. Oxidative stability of black cumin (Nigella sativa L.), Coriander (Coriandrum sativum L.) and Niger (Guizotia Abyssinica Cass.) crude seed oils upon stripping. Eur. J. Lipid Sci. Technol. 2004, 106, 35–43. [Google Scholar] [CrossRef]
- Lutterodt, H.; Luther, M.; Slavin, M.; Yin, J.-J.; Parry, J.; Gao, J.-M.; Yu, L. Fatty acid profile, thymoquinone content, oxidative stability, and antioxidant properties of cold-pressed black cumin seed oils. LWT-Food Sci. Technol. 2010, 43, 1409–1413. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Ostrowska-Ligęza, E.; Krygier, K. Impact of selected chemical characteristics of cold-pressed oils on their oxidative stability determined using the Rancimat and pressure differential scanning calorimetry method. Food Anal. Methods 2018, 11, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Novel Food Catalogue. Available online: https://ec.europa.eu/food/safety/novel-food/novel-food-catalogue_en (accessed on 25 April 2022).
- Bendini, A.; Barbieri, S.; Valli, E.; Buchecker, K.; Canavari, M.; Toschi, T.G. Quality evaluation of cold pressed sunflower oils by sensory and chemical analysis. Eur. J. Lipid Sci. Technol. 2011, 113, 1375–1384. [Google Scholar] [CrossRef]
- Wroniak, M.; Rękas, A.; Ratusz, K. Influence of impurities in raw material on sensory and physicochemical properties of cold-pressed rapeseed oil produced from conventionally and ecologically grown seeds. Acta Sci. Pol. Technol. Aliment. 2016, 15, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauferova, A.; Dordevic, D.; Jancikova, S.; Tremlova, B.; Kulawik, P. Fortified cold-pressed oils: The effect on sensory quality and functional properties. Separations 2021, 8, 55. [Google Scholar] [CrossRef]
- ISO 9936:2016; Animal and Vegetable Fats and Oils-Determination of Tocopherol and Tocotrienol Contents by High Performance Liquid Chromatography. ISO: Geneva, Switzerland, 2016.
- ISO 1228-1:2014; Animal and Vegetable Fats and Oils-Determination of Individual and Total Sterols Contents-Gas Chromatographic Method-Part 1. ISO: Geneva, Switzerland, 2014.
- Szydłowska-Czerniak, A.; Tułodziecka, A.; Momot, M.; Stawicka, B. Physicochemical, antioxidative, and sensory properties of refined rapeseed oils. J. Am. Oil Chem. Soc. 2019, 96, 405–419. [Google Scholar] [CrossRef]
- AOCS. Official Method Cd 12b-92: Oil stability index. In Official Method and Recommended Practices of the American Oil Chemist’s Society; AOCS Publishing: Champaing, IL, USA, 2017. [Google Scholar]
- ISO 27107:2010; Animal and Vegetable Fats and Oils-Determination of Peroxide Value-Potentiometric End-Point Determination. ISO: Geneva, Switzerland, 2010.
- ISO 660:1996; Animal and Vegetable Fats and Oils-Determination of Acid Value and Acidity. ISO: Geneva, Switzerland, 1996.
- ISO 662:1996; Animal and Vegetable Fats and Oils-Determination of Moisture and Volatile Matter Content. ISO: Geneva, Switzerland, 1996.
- ISO 5508:1996; Animal and Vegetable Fats and Oils-Analysis by Gas Chromatography of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 1996.
- ISO 5509:2000; Animal and Vegetable Fats and Oils-Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2000.
- ASTM E1627-94:2004; Standard Practice for Sensory Evaluation of Edible Oils and Fats. ASTM: West Conshohocken, PA, USA, 2004.
- DGF C-II 1, 09:2009; Quality Evaluation of Cold Pressed Oil. Section C-Fats. DGF: Frankfurt, Germany, 2009.
- ISO 11035:1994; Sensory Analysis-Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multi-Dimensional Approach. ISO: Geneva, Switzerland, 1994.
- ISO 8586-1:1996; Sensory Analysis-General Guidance for the Selection, Training and Monitoring of Assessors-Part 1: Selected Assessors. ISO: Geneva, Switzerland, 1996.
- ISO 8586-2:1996; Sensory Analysis-General Guidance for the Selection, Training and Monitoring of Assessors-Part 2: Expert Sensory Assessors. ISO: Geneva, Switzerland, 1996.
- Kostadinović Veličkovska, S.; Brühl, L.; Mitrev, S.; Mirhosseini, H.; Matthäus, B. Quality evaluation of cold-pressed edible oils from Macedonia. Eur. J. Lipid Sci. Technol. 2015, 117, 2023–2035. [Google Scholar] [CrossRef] [Green Version]
- Kiralan, M.; Özkan, G.; Bayrak, A.; Ramadan, M.F. Physicochemical properties and stability of black cumin (Nigella sativa) seed oil as affected by different extraction methods. Ind. Crops Prod. 2014, 57, 52–58. [Google Scholar] [CrossRef]
- Hassanein, M.M.; El-Shami, S.M.; El-Mallah, M.H. Investigation of lipids profiles of nigella, lupin and artichoke seed oils to be used as healthy oils. J. Oleo Sci. 2011, 60, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Grajzer, M.; Szmalcel, K.; Kuźmiński, Ł.; Witkowski, M.; Kulma, A.; Prescha, A. Characteristics and antioxidant potential of cold-pressed oils—possible strategies to improve oil stability. Foods 2020, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, N.K.; Abd Manap, M.Y.; Tan, C.P.; Muhialdin, B.J.; Alhelli, A.M.; Meor Hussin, A.S. The effects of different extraction methods on antioxidant properties, chemical composition, and thermal behavior of black seed (Nigella sativa L.) oil. J. Evid. Based Complement. Altern. Med. 2016, 2016, 6273817. [Google Scholar] [CrossRef] [Green Version]
- Sielicka, M.; Małecka, M.; Purłan, M. Comparison of the antioxidant capacity of lipid-soluble compounds in selected cold-pressed oils using photochemiluminescence assay (PCL) and DPPH method. Eur. J. Lipid Sci. Technol. 2014, 116, 388–394. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. A comprehensive review of the physicochemical, quality and nutritional properties of Nigella sativa oil. Food Rev. Int. 2019, 35, 342–362. [Google Scholar] [CrossRef]
- Codex Alimentarius. Codex Standard for Named Vegetable Oils. Codex-Stan 210-1999. Adopted in 1999. Revised in 2001, 2003, 2009, 2017, 2019. Amended in 2005, 2011, 2013, 2015, 2019, 2021. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 25 April 2022).
- Gotoh, N.; Wada, S. The importance of peroxide value in assessing food quality and food safety. J. Am. Oil Chem. Soc. 2006, 83, 473–474. [Google Scholar] [CrossRef]
- Mikołajczak, N.; Tańska, M.; Ogrodowska, D.; Czaplicki, S. Efficacy of canolol and guaiacol in the protection of cold-pressed oils being a dietary source linoleic acid against oxidative deterioration. Food Chem. 2022, 393, 133390. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 835/2011 of 19 August 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs. 2011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:215:0004:0008:EN:PDF (accessed on 25 April 2022).
- Badary, O.A.; Abd-Ellah, M.F.; El-Mahdy, M.A.; Salama, S.A.; Hamada, F.M. Anticlastogenic activity of thymoquinone against benzo(a)pyrene in mice. Food Chem. Toxicol. 2007, 45, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F.; Asker, M.M.S.; Tadros, M. Antiradical and antimicrobial properties of cold-pressed black cumin and cumin oils. Eur. Food Res. Technol. 2012, 234, 833–844. [Google Scholar] [CrossRef]
- Ozdemir, N.; Kantekin-Erdogan, M.N.; Tat, T.; Tekin, A. Effect of black cumin oil on the oxidative stability and sensory characteristics of mayonnaise. J. Food Sci. Technol. 2018, 55, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Brühl, L.; Matthäus, B.; Fehling, E.; Wiege, B.; Lehmann, B.; Luftmann, H.; Bergander, K.; Quiroga, K.; Scheipers, A.; Frank, O.; et al. Identification of bitter off-taste compounds in the stored cold pressed linseed oil. J. Agric. Food Chem. 2007, 55, 7864–7868. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rosales, F.; Ríos, J.J.; Gómez-Rey, M.L. Main polyphenols in the bitter taste of virgin olive oil. structural confirmation by on-line high-performance liquid chromatography electrospray ionization mass spectrometry. J. Agric. Food Chem. 2003, 51, 6021–6025. [Google Scholar] [CrossRef] [PubMed]



| Sensory Attributes | Definition | Reference Product | Score |
|---|---|---|---|
| OFI | The intensity of all flavor and taste attributes taken together | 1—Freshly refined rapeseed oil 8—Cold-pressed black cumin oil | 1; 8 |
| Color intensity | The intensity of brown color | Brown color intensity wheel | 0; 5; 10 |
| Sweet taste | A basic taste simulated by such as sugar | Fresh roasted cold-pressed oil | 9.5 |
| Bitter taste | A basic taste simulated by such substances as quinine and caffeine | 0.2% of caffeine in water or good quality of extra virgin oil | 7 |
| Herb-like flavor | The flavor reminiscent of herbs | Fresh cold-pressed black cumin oil | 6 |
| Flower-like flavor | The flavor reminiscent of fresh flowers | Freshly cold-pressed peanut oil | 8.5 |
| Medical-like flavor | The flavor reminiscent of medical, hospital, pharmacy | Cold-pressed black cumin oil stored for 24 months in room temperature | 7 |
| Fuel-like flavor | The flavor reminiscent of fuel | Bad quality cold-pressed black cumin oil | 9 |
| Spicy-like flavor | The flavor reminiscent of cumin | Cumin seed | 7 |
| Astringency | Shrinking or drying effect on the tongue surface caused by substances such as tannins | Slices of a green banana | 10 |
| Pungency | The biting sensation that can be perceived throughout the mouth cavity | A good quality extra virgin olive oil | 10 |
| Painty | The flavor reminiscent of oils such as linseed and rapeseed oils containing linolenic acid; it cannot be noted in non-linolenic acid oils such as peanut oil | Good quality canola oil aged 4–8 days at 60 °C or until PV = 10.0 meq O2/kg is reached | 10 |
| Score | Quality Level | Characteristic |
|---|---|---|
| 5 | Very good | Extra fresh taste characteristic for cold-pressed black cumin oil, high intensity taste with positive attributes such as herb-like, flower-like, spicy-like, and sweet taste. |
| 4 | Good | Positive tastes such as herb-like, flower-like, and sweet-like flavor at medium intensity. Slightly bitter and astringency is acceptable. |
| 3 | Fair | Negative taste attributes such as fuel-like, medical-like, and bitter taste and mouth feeling attributes such as pungency, astringency, and painty at low intensity were identified. |
| 2 | Bad | Negative taste attributes such as fuel-like, medical-like, and bitter taste and mouth feeling attributes such as pungency, astringency, and painty at moderate intensity were identified. |
| 1 | Very bad | Negative taste attributes such as fuel-like, medical-like, and bitter taste and mouth feeling attributes such as pungency, astringency, and painty at high/very high intensity were identified. |
| Compound | Content * ± SD (mg/100 g) | ||||||
|---|---|---|---|---|---|---|---|
| CPBCO1 | CPBCO2 | CPBCO3 | CPBCO4 | CPBCO5 | CPBCO6 | CPBCO7 | |
| α-Tocopherol | 6.9 ± 0.1 e | 0.6 ± 0.01 a | 3.0 ± 0.5 c | 6.5 ± 0.1 e | 1.6 ± 0.03 b | 5.5 ± 0.1 d | 3.4 ± 0.05 c |
| β-Tocopherol | 2.9 ± 0.05 c | <LOD | <LOD | <LOD | <LOD | 0.4 ± 0.02 b | 0.2 ± 0.01 a |
| γ-Tocopherol | <LOD | 1.3 ± 0.03 a | 1.1 ± 0.02 a | 35.1 ± 0.5 d | 2.3 ± 0.04 c | 1.3 ± 0.02 a | 1.8 ± 0.03 b |
| δ-Tocopherol | 0.6 ± 0.01 a | <LOD | <LOD | 0.7 ± 0.02 a | <LOD | <LOD | <LOD |
| Total tocopherols | 10.4 ± 0.1 e | 1.9 ± 0.03 a | 4.1 ± 0.05 b | 42.3 ± 0.5 f | 3.9 ± 0.05 b | 7.2 ± 0.01 d | 5.4 ± 0.06 c |
| Brassicasterol | 2 ± 0.03 b | <LOD | <LOD | 18 ± 0.3 c | <LOD | 1 ± 0.04 a | <LOD |
| Campesterol | 30 ± 0.5 d | 19 ± 0.3 a | 24 ± 0.4 b | 99 ± 1.5 e | 24 ± 0.4 b | 27 ± 0.4 c | 25 ± 0.4 b |
| Δ5-Avenasterol | 28 ± 0.4 a | 29 ± 0.4 a,b | 33 ± 0.5 c,d | 33 ± 0.6 c,d | 35 ± 0.5 d | 31 ± 0.7 b,c | 31 ± 0.5 b,c |
| β-Sitosterol | 110 ± 1.7 c | 81 ± 1.2 a | 118 ± 1.8 d | 182 ± 2.7 f | 103 ± 1.5 b | 121 ± 1.8 e | 119 ± 2.0 d,e |
| Total phytosterols | 208 ± 3 c | 159 ± 5 a | 211 ± 9 d | 372 ± 14 f | 201 ± 4 b | 222 ± 2 e | 212 ± 6 d |
| Oil Sample | Antioxidant Capacity * ± SD (µmol TE/100 g) | |||
|---|---|---|---|---|
| DPPH | ABTS | CUPRAC | FC | |
| CPBCO1 | 677.3 ± 16.0 d | 971.6 ± 20.9 c | 17,440.6 ± 749.8 f | 526.7 ± 23.7 d |
| CPBCO2 | 790.1 ± 11.4 f | 1465.0 ± 34.0 f | 19,823.3 ± 915.9 g | 643.7 ± 25.6 e |
| CPBCO3 | 276.9 ± 13.5 b | 385.9 ± 11.4 a | 975.3 ± 43.7 a | 206.8 ± 8.4 b |
| CPBCO4 | 355.8 ± 11.1 c | 653.9 ± 18.3 b | 7402.9 ± 379.0 d | 354.7 ± 15.3 c |
| CPBCO5 | 757.3 ± 13.2 e | 1270.8 ± 36.8 e | 8932.2 ± 549.4 e | 337.8 ± 13.4 c |
| CPBCO6 | 229.8 ± 5.3 a | 1098.6 ± 13.7 d | 2939.9 ± 413.9 b | 168.1 ± 12.8 a |
| CPBCO7 | 226.8 ± 6.7 a | 993.2 ± 32.2 c | 4144.0 ± 377.7 c | 177.0 ± 9.6 a |
| Parameter | Mean Value * ± SD | ||||||
|---|---|---|---|---|---|---|---|
| CPBCO1 | CPBCO2 | CPBCO3 | CPBCO4 | CPBCO5 | CPBCO6 | CPBCO7 | |
| IP (h) | 22.05 ± 0.40 e | 37.34 ± 0.20 g | 10.14 ± 0.40 c | 19.7 ± 0.40 d | 24.92 ± 0.20 f | 8.31 ± 0.30 a | 9.60 ± 0.40 b |
| PV (meq O2/kg) | 89.60 ± 0.01 f | 123.77 ± 0.56 g | 22.38 ± 0.01 b | 35.99 ± 0.01 d | 50.48 ± 0.02 e | 23.17 ± 0.02 c | 21.36 ± 0.05 a |
| AV (mg KOH/g) | 6.40 ± 0.06 a | 7.86 ± 0.02 b | 21.86 ± 0.08 f | 15.04 ± 0.08 d | 11.84 ± 0.09 c | 22.02 ± 0.08 g | 20.92 ± 0.10 e |
| WVC (%) | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.26 ± 0.01 c | 0.08 ± 0.00 b | 0.05 ± 0.00 a | 0.06 ± 0.00 a |
| B(a)P (µg/kg) | 0.01 ± 0.00 a | 3.76 ± 0.05 g | 0.38 ± 0.01 b | 0.43 ± 0.01 c | 0.85 ± 0.00 e | 1.10 ± 0.02 f | 0.70 ± 0.03 d |
| Chry (µg/kg) | 1.89 ± 0.01 e | 2.79 ± 0.01 f | 0.35 ± 0.01 b | 0.76 ± 0.01 c | 1.79 ± 0.09 d | 0.06 ± 0.00 a | 2.80 ± 0.07 f |
| B(a)A (µg/kg) | 16.05 ± 0.21 b | 36.98 ± 1.63 c | 3.40 ± 0.01 a | 14.48 ± 0.01 b | 16.47 ± 0.42 b | 3.20 ± 0.18 a | 4.00 ± 0.34 a |
| B(b)F (µg/kg) | 0.03 ± 0.00 a | 3.14 ± 0.08 e | 0.69 ± 0.00 c | 0.41 ± 0.02 b | 0.63 ± 0.03 c | 1.60 ± 0.07 d | 0.60 ± 0.01 c |
| ∑4PAHs (µg/kg) | 17.98 | 46.68 | 4.48 | 16.09 | 19.73 | 7.90 | 6.60 |
| Fatty Acid | Content * ± SD (%) | ||||||
|---|---|---|---|---|---|---|---|
| CPBCO1 | CPBCO2 | CPBCO3 | CPBCO4 | CPBCO5 | CPBCO6 | CPBCO7 | |
| C 16:0 | 12.02 ± 0.17 b,c | 13.03 ± 0.12 d | 12.06 ± 0.09 b,c | 8.27 ± 0.05 a | 12.39 ± 0.17 c | 11.98 ± 0.12 b | 12.45 ± 0.18 c |
| C 18:0 | 3.10 ± 0.03 a,b | 3.01 ± 0.04 a | 3.53 ± 0.05 c | 3.41 ± 0.04 c | 3.13 ± 0.09 a,b | 3.50 ± 0.04 c | 3.40 ± 0.07 c |
| C 20:0 | 0.20 ± 0.01 a | 0.20 ± 0.00 a | 0.26 ± 0.01 c | 0.20 ± 0.01 a | 0.21 ± 0.00 a | 0.28 ± 0.01 d | 0.24 ± 0.00 b,c |
| C 22:0 | 0.14 ± 0.00 c | 0.03 ± 0.00 a | 0.09 ± 0.00 b | 0.17 ± 0.01 c | 0.05 ± 0.00 a | 0.16 ± 0.00 c | <LOD |
| ΣSAFA | 15.91 | 16.76 | 16.23 | 12.14 | 16.12 | 16.12 | 16.25 |
| C 16:1 | 0.21 ± 0.01 b | 0.24 ± 0.01 c | 0.18 ± 0.01 a | 0.17 ± 0.00 a | 0.20 ± 0.01 b | 0.18 ± 0.00 a | 0.20 ± 0.01 b |
| C 18:1 | 26.05 ± 0.84 c | 24.74 ± 0.11 b | 26.39 ± 0.12 c | 31.01 ± 0.08 d | 23.89 ± 0.09 a | 26.11 ± 0.81 c | 25.32 ± 0.89 b |
| C 20:1 | 0.41 ± 0.02 b,c | 0.44 ± 0.01 c | 0.38 ± 0.01 a,b | 0.30 ± 0.00 a | 0.40 ± 0.01 b | 0.34 ± 0.00 a | 2.89 ± 0.04 d |
| ΣMUFA | 26.62 | 25.37 | 26.95 | 31.71 | 24.49 | 26.64 | 28.41 |
| C 18:2 | 57.08 ± 0.82 d | 57.63 ± 1.47 d | 56.53 ± 2.10 c | 32.80 ± 1.05 a | 59.08 ± 2.61 e | 56.49 ± 2.01 c | 55.06 ± 1.78 b |
| C 18:3 | 0.34 ± 0.01 b | 0.19 ± 0.00 a | 0.25 ± 0.02 a | 23.33 ± 0.90 d | 0.26 ± 0.01 a | 0.75 ± 0.03 c | 0.29 ± 0.01 a |
| ΣPUFA | 57.20 | 57.74 | 56.69 | 55.94 | 59.27 | 57.03 | 55.27 |
| Sensory Attribute | Mean Value * ± SD | ||||||
|---|---|---|---|---|---|---|---|
| CPBCO1 | CPBCO2 | CPBCO3 | CPBCO4 | CPBCO5 | CPBCO6 | CPBCO7 | |
| OSQ | 2.0 ± 0.0 a | 2.0 ± 0.1 a | 4.0 ± 0.1 c | 3.0 ± 0.1 b | 2.0 ± 0.0 a | 5.0 ± 0.2 d | 5.0 ± 0.1 d |
| OFI | 9.0 ± 0.3 c | 9.0 ± 0.1 c | 6.0 ± 0.2 a | 7.2 ± 0.3 b | 7.1 ± 0.2 b | 6.5 ± 0.1 a,b | 6.3 ± 0.2 a |
| Color intensity | 9.1 ± 0.2 e | 8.2 ± 0.2 d | 6.0 ± 0.2 a | 7.0 ± 0.1 b | 7.4 ± 0.3 b,c | 6.5 ± 0.2 a,b | 6.5 ± 0.1 a,b |
| Flavor | |||||||
| Herb-like | 1.3 ± 0.0 a | 1.1 ± 0.0 a | 7.8 ± 0.1 d,e | 5.0 ± 0.2 c | 2.5 ± 0.0 b | 8.3 ± 0.3 e | 7.2 ± 0.2 d |
| Flower-like | 0.0 ± 0.0 a | 1.0 ± 0.0 b | 5.6 ± 0.1 d | 3.0 ± 0.1 c | 2.5 ± 0.0 c | 8.3 ± 0.1 f | 7.2 ± 0.1 e |
| Medical-like | 8.0 ± 0.3 e | 7.5 ± 0.1 d | 3.4 ± 0.1 b | 4.0 ± 0.2 c | 7.8 ± 0.1 d,e | 3.0 ± 0.1 b | 1.7 ± 0.0 a |
| Fuel-like | 6.2 ± 0.2 b | 7.9 ± 0.1 c | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 7.2 ± 0.3 c | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| Spicy-like | 1.9 ± 0.0 a | 2.9 ± 0.1 b | 4.5 ± 0.2 c | 2.6 ± 0.1 b | 2.1 ± 0.1 a,b | 4.7 ± 0.1 c,d | 5.1 ± 0.2 d |
| Taste | |||||||
| Sweet | 1.1 ± 0.0 a | 0.9 ± 0.0 a | 3.7 ± 0.1 c | 1.5 ± 0.0 a,b | 1.2 ± 0.0 a | 4.3 ± 0.1 d | 3.1 ± 0.1 c |
| Bitter | 6.5 ± 0.2 c | 4.5 ± 0.1 b | 4.9 ± 0.1 b | 7.1 ± 0.3 d | 4.9 ± 0.1 b | 3.9 ± 0.1 a | 3.9 ± 0.2 a |
| Mouthfeeling | |||||||
| Astringency | 8.5 ± 0.4 d | 9.0 ± 0.4 d | 4.6 ± 0.1 a | 7.0 ± 0.3 c | 7.1 ± 0.2 c | 5.5 ± 0.2 b | 5.0 ± 0.1 a,b |
| Pungency | 7.1 ± 0.1 b | 9.1 ± 0.1 c | 6.2 ± 0.2 a | 7.3 ± 0.1 b | 6.0 ± 0.3 a | 7.0 ± 0.1 b | 6.7 ± 0.3 a,b |
| Painty | 7.0 ± 0.3 e | 6.0 ± 0.1 d | 2.0 ± 0.0 a | 4.2 ± 0.1 c | 7.2 ± 0.2 e | 3.0 ± 0.1 b | 3.0 ± 0.0 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydłowska-Czerniak, A.; Momot, M.; Stawicka, B.; Rabiej-Kozioł, D. Effects of the Chemical Composition on the Antioxidant and Sensory Characteristics and Oxidative Stability of Cold-Pressed Black Cumin Oils. Antioxidants 2022, 11, 1556. https://doi.org/10.3390/antiox11081556
Szydłowska-Czerniak A, Momot M, Stawicka B, Rabiej-Kozioł D. Effects of the Chemical Composition on the Antioxidant and Sensory Characteristics and Oxidative Stability of Cold-Pressed Black Cumin Oils. Antioxidants. 2022; 11(8):1556. https://doi.org/10.3390/antiox11081556
Chicago/Turabian StyleSzydłowska-Czerniak, Aleksandra, Monika Momot, Barbara Stawicka, and Dobrochna Rabiej-Kozioł. 2022. "Effects of the Chemical Composition on the Antioxidant and Sensory Characteristics and Oxidative Stability of Cold-Pressed Black Cumin Oils" Antioxidants 11, no. 8: 1556. https://doi.org/10.3390/antiox11081556
APA StyleSzydłowska-Czerniak, A., Momot, M., Stawicka, B., & Rabiej-Kozioł, D. (2022). Effects of the Chemical Composition on the Antioxidant and Sensory Characteristics and Oxidative Stability of Cold-Pressed Black Cumin Oils. Antioxidants, 11(8), 1556. https://doi.org/10.3390/antiox11081556