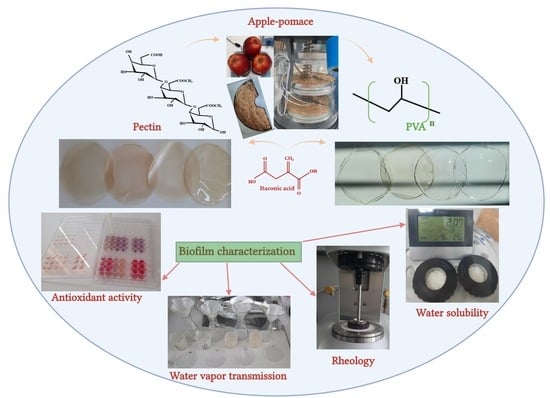
Development of Pectin and Poly(vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Apple Pomace Extracts
2.2.1. Freeze-Drying Process
2.2.2. Organic Acid Extraction and HPLC
2.2.3. Extraction and HPLC-DAD-MS-ESI + Analysis of Phenolic Compounds
2.3. Antioxidant Activity of Extracts before and after Lyophilization
2.4. Film Preparation
2.4.1. PVA Films
2.4.2. Pectin Films
2.5. Antimicrobial Activity of Extracts and Biofilm Solutions
2.5.1. Standard Strains
2.5.2. Preparation of Bacterial Strains
2.5.3. Determination of the Minimum Inhibitory Concentration (MIC)
2.6. Rheological Analyses
2.7. Biofilm Characterization
2.7.1. Physical Measurements
2.7.2. Water Vapor Transmission Rate
2.7.3. Water Solubility Test
2.8. Statistical Analyses
3. Results and Discussion
3.1. Phenolic and Organic Profile of Apple Pomace Extracts
3.2. Evaluation of Apple Pomace Extracts
3.2.1. Antioxidant Activity of Extracts before and after Lyophilization
3.2.2. Antimicrobial Activity of Extracts and Biofilms before and after Lyophilization
3.3. Rheological Measurements of Biofilm Solutions
3.4. Biofilm Characterization
3.4.1. Thickness, Diameter, Mass, and Density
3.4.2. Water Vapor Transmission Rate
3.4.3. Water Solubility of the Biofilms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nielsen, T.D.; Hasselbalch, J.; Holmberg, K.; Stripple, J. Politics and the plastic crisis: A review throughout the plastic life cycle. Wiley Interdiscip. Rev. Energy Environ. 2019, 9, e360. [Google Scholar] [CrossRef]
- Paletta, A.; Leal Filho, W.; Balogun, A.L.; Foschi, E.; Bonoli, A. Barriers and challenges to plastics valorisation in the context of a circular economy: Case studies from Italy. J. Clean. Prod. 2019, 241, 118149. [Google Scholar] [CrossRef]
- Jacob, J.; Lawal, U.; Thomas, S.; Valapa, R.B. Biobased Polymer Composite from Poly(lactic acid): Processing, Fabrication, and Characterization for Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128187951. [Google Scholar]
- Raddadi, N.; Fava, F. Biodegradation of oil-based plastics in the environment: Existing knowledge and needs of research and innovation. Sci. Total Environ. 2019, 679, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Blettler, M.C.M.; Wantzen, K.M. Threats Underestimated in Freshwater Plastic Pollution: Mini-Review. Water, Air, Soil Pollut. 2019, 230, 174. [Google Scholar] [CrossRef]
- PlasticsEurope Association of Plastics Manufacturers. PlasticsEurope Operation Clean Sweep ® Report. 2018. Available online: https://www.opcleansweep.eu/application/files/5416/3005/3212/PlasticsEurope_OCS_progress_repor.-2018.pdf (accessed on 8 August 2022).
- Teleky, B.-E.; Vodnar, D.C. Recent Advances in Biotechnological Itaconic Acid Production, and Application for a Sustainable Approach. Polymers 2021, 13, 3574. [Google Scholar] [CrossRef] [PubMed]
- Pascuta, M.S.; Varvara, R.; Teleky, B.-E.; Szabo, K.; Plamada, D.; Nemes, S.-A.; Mitrea, L.; Mărtau, G.A.; Ciont, C.; Călinoiu, L.-F.; et al. Polysaccharide-Based Edible Gels as Functional Ingredients. Gels 2022, 8, 524. [Google Scholar] [CrossRef]
- Chiloeches, A.; Cuervo-Rodríguez, R.; López-Fabal, F.; Fernández-García, M.; Echeverría, C.; Muñoz-Bonilla, A. Antibacterial and compostable polymers derived from biobased itaconic acid as environmentally friendly additives for biopolymers. Polym. Test. 2022, 109, 107541. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Socaciu, M.-I.; Semeniuc, C.; Vodnar, D. Edible Films and Coatings for Fresh Fish Packaging: Focus on Quality Changes and Shelf-life Extension. Coatings 2018, 8, 366. [Google Scholar] [CrossRef]
- Pascuta, M.S.; Vodnar, D.C. Nanocarriers for sustainable active packaging: An overview during and post COVID-19. Coatings 2022, 12, 102. [Google Scholar] [CrossRef]
- Teleky, B.E.; Vodnar, D.C. Biomass-derived production of itaconic acid as a building block in specialty polymers. Polymers 2019, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Cottet, C.; Salvay, G.; Peltzer, M.A. Incorporation of Poly(Itaconic Acid) with Quaternized Thiazole Groups on Gelatin-Based Films for Antimicrobial-Active Food Packaging. Polymers 2021, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Nemes, S.A.; Szabo, K. Applicability of Agro-Industrial By-Products in Intelligent Food Packaging. Coatings 2020, 10, 550. [Google Scholar] [CrossRef]
- Coelho, C. Itaconate or how i learned to stop avoiding the study of immunometabolism. PLoS Pathog. 2022, 18, e1010361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Y.; Zhao, Y.; Tao, L.; Liu, H.; Dong, W.; Yang, G.; Li, L. Effects of dietary supplementation with itaconic acid on the growth performance, nutrient digestibility, slaughter variables, blood biochemical parameters, and intestinal morphology of broiler chickens. Poult. Sci. 2022, 101, 101732. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Antimicrobial packaging efficiency of ZnO-SiO2 nanocomposites infused into PVA/CS film for enhancing the shelf life of food products. Food Packag. Shelf Life 2020, 25, 100523. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y.; Davies, I.J.; Barbhuiya, S. PVA, PVA Blends, and Their Nanocomposites for Biodegradable Packaging Application. Polym. Plast. Technol. Eng. 2017, 56, 1307–1344. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.E.; Mitrea, L.; Călinoiu, L.F.; Martău, G.A.; Simon, E.; Varvara, R.A.; Vodnar, D.C. Active packaging-poly (vinyl alcohol) films enriched with tomato by-products extract. Coatings 2020, 10, 141. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.-F.F.; Martău, G.-A.; Szabo, K.; Teleky, B.-E.E.; Mureșan, V.; Rusu, A.-V.V.; Socol, C.-T.T.; Vodnar, D.-C.C.; Mărtau, G.A.; et al. Poly(vinyl alcohol)-based biofilms plasticized with polyols and colored with pigments extracted from tomato by-products. Polymers 2020, 12, 532. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Varvara, R.A.; Szabo, K.; Vodnar, D.C. 3D food printing: Principles of obtaining digitally-designed nourishment. Nutrients 2021, 13, 3617. [Google Scholar] [CrossRef] [PubMed]
- Thiraviam, V.; Mahejibin, K. Role of Pectin in Food Processing and Food Packaging. In Pectins—Extraction, Purification, Characterization and Applications; IntechOpen: London, UK, 2019; p. 178. [Google Scholar]
- Mitrea, L.; Nemes, S.-A.; Szabo, K.; Teleky, B.-E.; Vodnar, D.-C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association With Neurological and Psychiatric Disorders. Front. Med. 2022, 9, 822190. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.C.M.; Arraibi, A.A.; Ferreira, I.C.F.R. Bioactive and functional compounds in apple pomace from juice and cider manufacturing: Potential use in dermal formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2019; Volume 91, pp. 157–225. [Google Scholar]
- Precup, G.; Mitrea, L.; Nemes, A.; Călinoiu, L.-F.; Martău, G.-A.; Teleky, B.E.; Coman, V.; Vodnar, D.C. Food processing by-products and molecular gastronomy. In Gastronomy and Food Science; Academic Press: Cambridge, MA, USA, 2021; pp. 137–164. [Google Scholar]
- Martau, G.A.; Teleky, B.-E.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Apple Pomace as a Sustainable Substrate in Sourdough Fermentation. Front. Microbiol. 2021, 12, 3850. [Google Scholar] [CrossRef]
- da Silva, L.C.; Viganó, J.; de Souza Mesquita, L.M.; Dias, A.L.B.; de Souza, M.C.; Sanches, V.L.; Chaves, J.O.; Pizani, R.S.; Contieri, L.S.; Rostagno, M.A. Recent advances and trends in extraction techniques to recover polyphenols compounds from apple by-products. Food Chem. X 2021, 12, 100133. [Google Scholar] [CrossRef]
- Heras-Ramírez, M.E.; Quintero-Ramos, A.; Camacho-Dávila, A.A.; Barnard, J.; Talamás-Abbud, R.; Torres-Muñoz, J.V.; Salas-Muñoz, E. Effect of Blanching and Drying Temperature on Polyphenolic Compound Stability and Antioxidant Capacity of Apple Pomace. Food Bioprocess Technol. 2012, 5, 2201–2210. [Google Scholar] [CrossRef]
- González, C.M.; Llorca, E.; Quiles, A.; Hernando, I.; Moraga, G. Water sorption and glass transition in freeze-dried persimmon slices. Effect on physical properties and bioactive compounds. LWT 2020, 130, 109633. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.; Ranga, F.; Roman, I.; Khaoula, H.; Boudaya, E.; Ltaief, A.B.; Aouani, W.; Thiamrat, M.; Vodnar, D.C. Carotenoid Recovery from Tomato Processing By-Products through Green Chemistry. Molecules 2022, 27, 3771. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Enig, M.; Brook, I.; Elliott, T.B. Minimum inhibitory concentrations of herbal essential oils and monolaurin for gram-positive and gram-negative bacteria. Mol. Cell. Biochem. 2005, 272, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef]
- Vodnar, D.C. Inhibition of Listeria monocytogenes ATCC 19115 on ham steak by tea bioactive compounds incorporated into chitosan-coated plastic films. Chem. Cent. J. 2012, 6, 2–7. [Google Scholar] [CrossRef]
- Mitrea, L.; Teleky, B.-E.; Leopold, L.-F.; Nemes, S.-A.; Plamada, D.; Dulf, F.V.; Pop, I.-D.; Vodnar, D.C. The physicochemical properties of five vegetable oils exposed at high temperature for a short-time-interval. J. Food Compos. Anal. 2022, 106, 104305. [Google Scholar] [CrossRef]
- Teleky, B.-E.; Martău, G.-A.; Vodnar, D.-C. Physicochemical Effects of Lactobacillus plantarum and Lactobacillus casei Cocultures on Soy–Wheat Flour Dough Fermentation. Foods 2020, 9, 1894. [Google Scholar] [CrossRef]
- Precup, G.; Teleky, B.-E.; Ranga, F.; Vodnar, D.C. Assessment of Physicochemical and Rheological Properties of Xylo-Oligosaccharides and Glucose-Enriched Doughs Fermented with BB-12. Biology 2022, 11, 553. [Google Scholar] [CrossRef]
- Wiedenmann, V.; Oehlke, K.; van der Schaaf, U.; Koivula, H.M.; Mikkonen, K.S.; Karbstein, H.P. Emulsifier Composition of Solid Lipid Nanoparticles (SLN) Affects Mechanical and Barrier Properties of SLN-Protein Composite Films. J. Food Sci. 2019, 84, 3642–3652. [Google Scholar] [CrossRef]
- Maizura, M.; Fazilah, A.; Norziah, M.H.; Karim, A.A. Antibacterial activity and mechanical properties of partially hydrolyzed sago starch-alginate edible film containing lemongrass oil. J. Food Sci. 2007, 72, C324–C330. [Google Scholar] [CrossRef] [PubMed]
- Saputri, A.E.; Praseptiangga, D.; Rochima, E.; Panatarani, C.; Joni, I.M. Mechanical and solubility properties of bio-nanocomposite film of semi refined kappa carrageenan/ZnO nanoparticles. AIP Conf. Proc. 2018, 1927, 030040. [Google Scholar] [CrossRef]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Saha, A.; Tyagi, S.; Gupta, R.K.; Tyagi, Y.K. Natural gums of plant origin as edible coatings for food industry applications. Crit. Rev. Biotechnol. 2017, 37, 959–973. [Google Scholar] [CrossRef]
- Huang, H.; Wang, L.; Bi, F.; Xiang, X. Combined Application of Malic Acid and Lycopene Maintains Content of Phenols, Antioxidant Activity, and Membrane Integrity to Delay the Pericarp Browning of Litchi Fruit During Storage. Front. Nutr. 2022, 9, 849385. [Google Scholar] [CrossRef]
- Anyasi, T.A.; Jideani, A.I.O.; Edokpayi, J.N.; Anokwuru, C.P. Organic Acids—Characteristics, Properties, and Synthesis; Vargas, C., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; ISBN 9781631172557. [Google Scholar]
- Priyadarshi, R.; Sauraj, B.; Kumar, Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef]
- Rechia, L.M.; de Jesus Morona, J.B.; Zepon, K.M.; Soldi, V.; Kanis, L.A. Mechanical properties and total hydroxycinnamic derivative release of starch/glycerol/Melissa officinalis extract films. Braz. J. Pharm. Sci. 2010, 46, 491–497. [Google Scholar] [CrossRef]
- Bonnenfant, C.; Gontard, N.; Aouf, C. Extending biopolyesters circularity by using natural stabilizers: A review on the potential of polyphenols to enhance Poly(hydroxyalkanoates) thermal stability while preserving its biodegradability. Polym. Test. 2022, 110, 107561. [Google Scholar] [CrossRef]
- Jiao, W.; Shu, C.; Li, X.; Cao, J.; Fan, X.; Jiang, W. Preparation of a chitosan-chlorogenic acid conjugate and its application as edible coating in postharvest preservation of peach fruit. Postharvest Biol. Technol. 2019, 154, 129–136. [Google Scholar] [CrossRef]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Heredia, J.B. Effect of dietary intake of phenolic compounds from mango peel extract on growth, lipid peroxidation and antioxidant enzyme activities in zebrafish (Danio rerio). Lat. Am. J. Aquat. Res. 2019, 47, 602–611. [Google Scholar] [CrossRef]
- Yu, L.; Shi, H. Effect of two mulberry (Morus alba L.) leaf polyphenols on improving the quality of fresh-cut cantaloupe during storage. Food Control 2021, 121, 107624. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W. Fabrication of Quercetin-Loaded Biopolymer Films as Functional Packaging Materials. ACS Appl. Polym. Mater. 2021, 3, 2131–2137. [Google Scholar] [CrossRef]
- Gonçalves, B.; Moeenfard, M.; Rocha, F.; Alves, A.; Estevinho, B.N.; Santos, L. Microencapsulation of a Natural Antioxidant from Coffee—Chlorogenic Acid (3-Caffeoylquinic Acid). Food Bioprocess Technol. 2017, 10, 1521–1530. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Toşa, M.I. Total Phenolic Contents, Antioxidant Activities, and Lipid Fractions from Berry Pomaces Obtained by Solid-State Fermentation of Two Sambucus Species with Aspergillus niger. J. Agric. Food Chem. 2015, 63, 3489–3500. [Google Scholar] [CrossRef]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar]
- Ahmed, M.; Ali, A.; Sarfraz, A.; Hong, Q.; Boran, H. Effect of Freeze-Drying on Apple Pomace and Pomegranate Peel Powders Used as a Source of Bioactive Ingredients for the Development of Functional Yogurt. J. Food Qual. 2022, 2022, 3327401. [Google Scholar] [CrossRef]
- Rana, S.; Gupta, S.; Rana, A.; Bhushan, S. Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient. Food Sci. Hum. Wellness 2015, 4, 180–187. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Călinoiu, L.F.; Dulf, F.V.; Ştefănescu, B.E.; Crişan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef]
- Chahardoli, A.; Jalilian, F.; Memariani, Z.; Farzaei, M.H.; Shokoohinia, Y. Chapter 26—Analysis of Organic Acids; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128164556. [Google Scholar]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesic, A.R.; Trifunovic, S.S.; Grujic, A.S.; Velickovic, S.J.; Antonovic, D.G. Complexation of amidated pectin with poly(itaconic acid) as a polycarboxylic polymer model compound. Carbohydr. Res. 2011, 346, 2463–2468. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, K.; Dabrowska, A.; Kaczmarek, H. Rheological properties of pectin, poly(vinyl alcohol) and their blends in aqueous solutions. E-Polymers 2012, 12. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.-F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Ştefănescu, B.-E.; Pop, I.-D.; Vodnar, D.-C. Isolated microorganisms for bioconversion of biodiesel-derived glycerol into 1,3-Propanediol. Bull. UASVM Food Sci. Technol. 2017, 74, 43–49. [Google Scholar] [CrossRef]
- Ding, J.; Chen, S.C.; Wang, X.L.; Wang, Y.Z. Preparation and rheological behaviors of thermoplastic poly(vinyl alcohol) modified by lactic acid. Ind. Eng. Chem. Res. 2011, 50, 9123–9130. [Google Scholar] [CrossRef]
- WHO Principles and Methods for the Risk Assessment of Chemicals in Food Chapter 6: Dietary Exposure Assessment of Chemicals in Food. In WHO: Geneva; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–98.
- Rodsamran, P.; Sothornvit, R. Lime peel pectin integrated with coconut water and lime peel extract as a new bioactive film sachet to retard soybean oil oxidation. Food Hydrocoll. 2019, 97, 105173. [Google Scholar] [CrossRef]
- Narasagoudr, S.S.; Hegde, V.G.; Vanjeri, V.N.; Chougale, R.B.; Masti, S.P. Ethyl vanillin incorporated chitosan/poly(vinyl alcohol) active films for food packaging applications. Carbohydr. Polym. 2020, 236, 116049. [Google Scholar] [CrossRef]
- Bhat, V.G.; Narasagoudr, S.S.; Masti, S.P.; Chougale, R.B.; Shanbhag, Y. Hydroxy citric acid cross-linked chitosan/guar gum/poly(vinyl alcohol) active films for food packaging applications. Int. J. Biol. Macromol. 2021, 177, 166–175. [Google Scholar] [CrossRef]
- Zia, J.; Paul, U.C.; Heredia-Guerrero, J.A.; Athanassiou, A.; Fragouli, D. Low-density polyethylene/curcumin melt extruded composites with enhanced water vapor barrier and antioxidant properties for active food packaging. Polymer 2019, 175, 137–145. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Kirimura, K.; Honda, Y.; Hattori, T. Gluconic and Itaconic Acids, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, ISBN 9780080885049. [Google Scholar]
- Ghaderi, J.; Hosseini, S.F.; Keyvani, N.; Gómez-Guillén, M.C. Polymer blending effects on the physicochemical and structural features of the chitosan/poly(vinyl alcohol)/fish gelatin ternary biodegradable films. Food Hydrocoll. 2019, 95, 122–132. [Google Scholar] [CrossRef]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Biodegradable and water resistant poly(vinyl) alcohol (PVA)/starch (ST)/glycerol (GL)/halloysite nanotube (HNT) nanocomposite films for sustainable food packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef]
- Guadarrama-Lezama, A.Y.; Castaño, J.; Velázquez, G.; Carrillo-Navas, H.; Alvarez-Ramírez, J. Effect of nopal mucilage addition on physical, barrier and mechanical properties of citric pectin-based films. J. Food Sci. Technol. 2018, 55, 3739–3748. [Google Scholar] [CrossRef] [PubMed]
- Giancone, T.; Torrieri, E.; Pierro, P.D.; Cavella, S.; Giosafatto, C.V.L.; Masi, P. Effect of Surface Density on the Engineering Properties of High Methoxyl Pectin-Based Edible Films. Food Bioprocess Technol. 2011, 4, 1228–1236. [Google Scholar] [CrossRef] [Green Version]





| Apple Treatment (mg/g)/Organic Acid | Malic Acid | Citric Acid | Succinic Acid | Fumaric Acid | Total |
|---|---|---|---|---|---|
| Frozen | 2.671 ± 0.076 ** | 0.840 ± 0.005 * | 0.085 ± 0.006 * | 0.001 ± 0.000 * | 3.597 ± 0.066 ** |
| Lyophilized | 14.730 ± 0.124 | 4.556 ± 0.109 | 0.530 ± 0.023 | 0.012 ± 0.001 | 19.828 ± 0.211 |
| Peak No. | Rt (min) | UV λmax (nm) | [M+H]+ (m/z) | Compound | Subclass | Frozen | Lyophilized |
|---|---|---|---|---|---|---|---|
| 1 | 3.25 | 280 | 333 | Gallic acid-glucoside | Hydroxybenzoic acid | 45.47 ± 0.07 ** | 106.62 ± 0.14 |
| 2 | 10.70 | 280, 520 | 449, 287 | Cyanidin-glucoside | Anthocyanin | 20.29 ± 0.00 ** | 78.47 ± 0.18 |
| 3 | 12.45 | 323 | 355 | 5-Caffeoylquinic acid (Chlorogenic acid) | Hydroxycinnamic acid | 156.71 ± 0.09 ** | 1173.13 ± 0.19 |
| 4 | 13.09 | 280 | 579, 291 | Procyanidin dimmer | Flavanol | 124.70 ± 0.10 ** | 723.07 ± 0.06 |
| 5 | 13.62 | 322 | 343 | Caffeic acid-glucoside | Hydroxycinnamic acid | 42.10 ± 0.03 ** | 202.74 ± 0.29 |
| 6 | 13.74 | 280 | 291 | Epicatechin | Flavanol | 190.36 ± 0.04 ** | 736.21 ± 0.44 |
| 7 | 14.23 | 316 | 339 | p-Coumaroylquinic acid | Hydroxycinnamic acid | 45.41 ± 0.01 ** | 222.40 ± 0.04 |
| 8 | 15.53 | 259, 348 | 611, 303 | Quercetin-rutinoside (Rutin) | Flavonol | 11.85 ± 0.16 ** | 34.66 ± 0.07 |
| 9 | 16.06 | 259, 350 | 465, 303 | Quercetin-glucoside (Isoquercitrin) | Flavonol | 217.84 ± 0.08 ** | 358.58 ± 0.07 |
| 10 | 16.73 | 260, 348 | 435, 303 | Quercetin-arabinoside (Avicularin) | Flavonol | 79.68 ± 0.23 ** | 11.58 ± 0.09 |
| 11 | 17.01 | 285 | 569, 275 | Phloretin-xylosyl-glucoside | Dihydrochalcone | 34.61 ± 0.09 ** | 201.59 ± 0.09 |
| 12 | 17.21 | 260, 349 | 551, 303 | Quercetin-(malonyl)-glucoside | Flavonol | 129.17 ± 0.09 ** | 118.88 ± 0.04 |
| 13 | 17.39 | 260, 347 | 449, 303 | Quercetin-rhamnoside (Quercitrin) | Flavonol | 88.46 ± 0.06 ** | 164.07 ± 0.09 |
| 14 | 18.35 | 287 | 437, 275 | Phloretin-glucoside (Phloridzin) | Dihydrochalcone | 114.18 ± 0.03 ** | 720.39 ± 0.09 |
| Total Phenolics | 1300.82 ± 0.10 ** | 4952.40 ± 0.08 |
| 1-AP-PE | 9.70 ± 0.078 ** | μM Trolox/100 g fresh Weight |
| 2-Lyophilized AP-PE | 67.45 ± 0.28 ** | μM Trolox/100 g dry weight |
| 3-AP-OE | 78.61 ± 0.24 ** | μM Trolox/100 g fresh weight |
| 4-Lyophilized AP-OE | 166.69 ± 0.47 ** | μM Trolox/100 g dry weight |
| Films | Thickness/µm | Diameter/mm | Mass/g | Density/g·cm−3 |
|---|---|---|---|---|
| PVA+Gly | 92 ± 23 | 53.54 ± 0.29 | 0.19 ± 0.00 | 1.30 ± 0.16 |
| PVA+Gly+IA | 84 ± 8 NS | 53.40 ± 0.03 NS | 0.24 ± 0.01 ** | 1.55 ± 0.10 NS |
| PVA+Gly+IA+OE | 74 ± 10 * | 53.87 ± 0.29 NS | 0.22 ± 0.01 * | 1.33 ± 0.02 * |
| PVA+Gly+IA+PE | 74 ± 8 * | 53.95 ± 0.39 NS | 0.26 ± 0.01 ** | 1.55 ± 0.10 ** |
| PEC+ Gly | 48 ± 17 | 53.35 ± 0.13 | 0.12 ± 0.01 | 1.21 ± 0.29 |
| PEC+Gly+IA | 53 ± 5 NS | 53.22 ± 0.22 NS | 0.17 ± 0.00 NS | 1.46 ± 0.05 NS |
| PEC+Gly+IA+OE | 75 ± 24 ** | 53.87 ± 0.29 ** | 0.17 ± 0.01 NS | 1.05 ± 0.21 NS |
| PEC+Gly+IA+PE | 55 ± 5 NS | 53.39 ± 0.25 NS | 0.20 ± 0.02 * | 1.63 ± 0.14 NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teleky, B.-E.; Mitrea, L.; Plamada, D.; Nemes, S.A.; Călinoiu, L.-F.; Pascuta, M.S.; Varvara, R.-A.; Szabo, K.; Vajda, P.; Szekely, C.; et al. Development of Pectin and Poly(vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants 2022, 11, 1729. https://doi.org/10.3390/antiox11091729
Teleky B-E, Mitrea L, Plamada D, Nemes SA, Călinoiu L-F, Pascuta MS, Varvara R-A, Szabo K, Vajda P, Szekely C, et al. Development of Pectin and Poly(vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants. 2022; 11(9):1729. https://doi.org/10.3390/antiox11091729
Chicago/Turabian StyleTeleky, Bernadette-Emőke, Laura Mitrea, Diana Plamada, Silvia Amalia Nemes, Lavinia-Florina Călinoiu, Mihaela Stefana Pascuta, Rodica-Anita Varvara, Katalin Szabo, Patricia Vajda, Cristian Szekely, and et al. 2022. "Development of Pectin and Poly(vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants" Antioxidants 11, no. 9: 1729. https://doi.org/10.3390/antiox11091729
APA StyleTeleky, B. -E., Mitrea, L., Plamada, D., Nemes, S. A., Călinoiu, L. -F., Pascuta, M. S., Varvara, R. -A., Szabo, K., Vajda, P., Szekely, C., Martău, G. -A., Elemer, S., Ranga, F., & Vodnar, D. -C. (2022). Development of Pectin and Poly(vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants, 11(9), 1729. https://doi.org/10.3390/antiox11091729