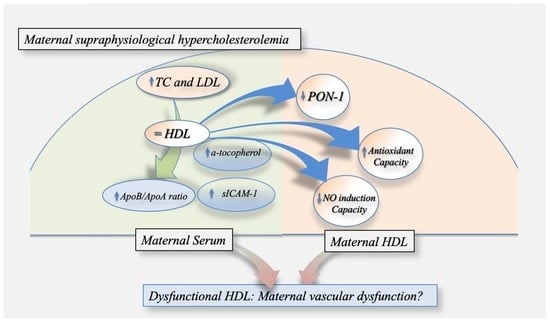
Maternal Supraphysiological Hypercholesterolemia Is Accompanied by Shifts in the Composition and Anti-Atherogenic Functions of Maternal HDL along with Maternal Cardiovascular Risk Markers at Term of Pregnancy
Abstract
:1. Introduction
2. Methods
2.1. Study Groups
2.2. Determination of Maternal Cholesterol and Triglyceride Levels
2.3. HDL Isolation
2.4. Human Umbilical Vein Endothelial Cell Culture
2.5. Endothelial Cell Line Culture
2.6. Protein Quantification and Western Blot
2.7. Lipid Determination Assays
2.8. Intracellular Reactive Oxygen Species (ROS) Determination
2.9. PON1 Enzymatic Activity
2.10. Alpha-Tocopherol Levels
2.11. Cholesterol Efflux Capacity
2.12. Endothelial Activation Assay
2.13. Nitric Oxide Determination
2.14. Inflammation Marker Determination
2.15. Apolipoprotein Determination
2.16. Endothelial Dysfunction Marker Determination
2.17. Determination of Total Antioxidant Capacity in Plasma
2.18. Cardiovascular Risk Markers
2.18.1. ApoB/ApoAI Ratio
2.18.2. Atherogenic Index of Plasma (AIP)
2.19. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Participants
3.2. Composition of Maternal HDL from MPH and MSPH Pregnancies
3.3. Biological Activities of Maternal HDL from MPH and MSPH Pregnancies
3.4. Cytokine Levels in Maternal Serum
3.5. Apolipoprotein Levels in Maternal Serum
3.6. Markers of Endothelial Dysfunction, Antioxidant Capacity, and Cardiovascular Risk in Maternal Serum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Napoli, C.; D’Armiento, F.P.; Mancini, F.P.; Postiglione, A.; Witztum, J.L.; Palumbo, G.; Palinski, W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J. Clin. Invest. 1997, 100, 2680–2690. [Google Scholar] [CrossRef]
- Leiva, A.; Salsoso, R.; Saez, T.; Sanhueza, C.; Pardo, F.; Sobrevia, L. Cross-sectional and longitudinal lipid determination studies in pregnant women reveal an association between increased maternal LDL cholesterol concentrations and reduced human umbilical vein relaxation. Placenta 2015, 36, 895–902. [Google Scholar] [CrossRef]
- Wiznitzer, A.; Mayer, A.; Novack, V.; Sheiner, E.; Gilutz, H.; Malhotra, A.; Novack, L. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: A population-based study. Am. J. Obstet. Gynecol. 2009, 201, 482.e481–482.e488. [Google Scholar] [CrossRef]
- Montes, A.; Walden, C.E.; Knopp, R.H.; Cheung, M.; Chapman, M.B.; Albers, J.J. Physiologic and supraphysiologic increases in lipoprotein lipids and apoproteins in late pregnancy and postpartum. Possible markers for the diagnosis of “prelipemia”. Arteriosclerosis 1984, 4, 407–417. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, S.; Ma, W.-W.; Cai, X.-P.; Le, Z.-Y.; Xiao, R.; Zhou, Q.; Yu, H.-L. Modulation of cholesterol transport by maternal hypercholesterolemia in human full-term placenta. PLoS ONE 2017, 12, e0171934. [Google Scholar] [CrossRef]
- Leiva, A.; de Medina, C.D.; Salsoso, R.; Sáez, T.; San Martín, S.; Abarzúa, F.; Farías, M.; Guzmán-Gutiérrez, E.; Pardo, F.; Sobrevia, L. Maternal Hypercholesterolemia in Pregnancy Associates With Umbilical Vein Endothelial DysfunctionSignificance: Role of Endothelial Nitric Oxide Synthase and Arginase II. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2444–2453. [Google Scholar] [CrossRef]
- Fuenzalida, B.; Sobrevia, B.; Cantin, C.; Carvajal, L.; Salsoso, R.; Gutiérrez, J.; Contreras-Duarte, S.; Sobrevia, L.; Leiva, A. Maternal supraphysiological hypercholesterolemia associates with endothelial dysfunction of the placental microvasculature. Sci. Rep. 2018, 8, 7690. [Google Scholar] [CrossRef]
- Leiva, A.; Fuenzalida, B.; Salsoso, R.; Barros, E.; Toledo, F.; Gutiérrez, J.; Pardo, F.; Sobrevia, L. Tetrahydrobiopterin Role in human umbilical vein endothelial dysfunction in maternal supraphysiological hypercholesterolemia. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 536–544. [Google Scholar] [CrossRef]
- Liguori, A.; D’Armiento, F.P.; Palagiano, A.; Balestrieri, M.L.; Williams-Ignarro, S.; de Nigris, F.; Lerman, L.O.; D’Amora, M.; Rienzo, M.; Fiorito, C.; et al. Effect of gestational hypercholesterolaemia on omental vasoreactivity, placental enzyme activity and transplacental passage of normal and oxidised fatty acids. BJOG 2007, 114, 1547–1556. [Google Scholar] [CrossRef]
- Fuenzalida, B.; Cantin, C.; Kallol, S.; Carvajal, L.; Pastén, V.; Contreras-Duarte, S.; Albrecht, C.; Gutierrez, J.; Leiva, A. Cholesterol uptake and efflux are impaired in human trophoblast cells from pregnancies with maternal supraphysiological hypercholesterolemia. Sci. Rep. 2020, 10, 5264. [Google Scholar] [CrossRef]
- Napoli, C.; Glass, C.K.; Witztum, J.L.; Deutsch, R.; D’Armiento, F.P.; Palinski, W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet 1999, 354, 1234–1241. [Google Scholar] [CrossRef]
- Cacciatore, F.; Bruzzese, G.; Abete, P.; Russo, G.; Palinski, W.; Napoli, C. Maternal hypercholesterolaemia during pregnancy affects severity of myocardial infarction in young adults. Eur. J. Prev. Cardiol. 2022, 29, 758–765. [Google Scholar] [CrossRef]
- Cantin, C.; Garchitorena, M.J.; Escalona, R.; Carvajal, J.A.; Illanes, S.E.; Gutierrez, J.; Leiva, A. Increased Circulating Levels of PCSK9 and Pro-Atherogenic Lipoprotein Profile in Pregnant Women with Maternal Supraphysiological Hypercholesterolemia. Antioxidants 2022, 11, 869. [Google Scholar] [CrossRef]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar] [CrossRef]
- Cantin, C.; Arenas, G.; San Martin, S.; Leiva, A. Effects of lipoproteins on endothelial cells and macrophages function and its possible implications on fetal adverse outcomes associated to maternal hypercholesterolemia during pregnancy. Placenta 2021, 106, 79–87. [Google Scholar] [CrossRef]
- Silliman, K.; Tall, A.R.; Kretchmer, N.; Forte, T.M. Unusual high-density lipoprotein subclass distribution during late pregnancy. Metabolism 1993, 42, 1592–1599. [Google Scholar] [CrossRef]
- Zeljkovic, A.; Vekic, J.; Spasic, S.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V.; Gojkovic, T.; Ardalic, D.; Mandic-Markovic, V.; Cerovic, N.; Mikovic, Z. Changes in LDL and HDL subclasses in normal pregnancy and associations with birth weight, birth length and head circumference. Matern. Child Health J. 2013, 17, 556–565. [Google Scholar] [CrossRef]
- Melchior, J.T.; Swertfeger, D.K.; Morris, J.; Street, S.E.; Warshak, C.R.; Welge, J.A.; Remaley, A.T.; Catov, J.M.; Davidson, W.S.; Woollett, L.A. Pregnancy is accompanied by larger high density lipoprotein particles and compositionally distinct subspecies. J. Lipid Res. 2021, 62, 100107. [Google Scholar] [CrossRef]
- Contreras-Duarte, S.; Escalona-Rivano, R.; Cantin, C.; Valdivia, P.; Zapata, D.; Carvajal, L.; Brito, R.; Cerda, Á.; Illanes, S.; Gutiérrez, J.; et al. Small extracellular vesicles from pregnant women with maternal supraphysiological hypercholesterolemia impair endothelial cell function in vitro. Vascul. Pharmacol. 2023, 150, 107174. [Google Scholar] [CrossRef]
- Camont, L.; Chapman, M.J.; Kontush, A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol. Med. 2011, 17, 594–603. [Google Scholar] [CrossRef]
- Kontush, A. HDL-mediated mechanisms of protection in cardiovascular disease. Cardiovasc. Res. 2014, 103, 341–349. [Google Scholar] [CrossRef]
- Sreckovic, I.; Birner-Gruenberger, R.; Besenboeck, C.; Miljkovic, M.; Stojakovic, T.; Scharnagl, H.; Marsche, G.; Lang, U.; Kotur-Stevuljevic, J.; Jelic-Ivanovic, Z.; et al. Gestational diabetes mellitus modulates neonatal high-density lipoprotein composition and its functional heterogeneity. Biochim. Biophys. Acta 2014, 1841, 1619–1627. [Google Scholar] [CrossRef]
- Abuarqoub, D.; Aslam, N.; Zaza, R.; Jafar, H.; Zalloum, S.; Atoom, R.; Alshaer, W.; Al-Mrahleh, M.; Awidi, A. The Immunomodulatory and Regenerative Effect of Biodentine™ on Human THP-1 Cells and Dental Pulp Stem Cells: In Vitro Study. Biomed. Res. Int. 2022, 2022, 2656784. [Google Scholar] [CrossRef]
- Boiko, A.S.; Mednova, I.A.; Kornetova, E.G.; Semke, A.V.; Bokhan, N.A.; Loonen, A.J.M.; Ivanova, S.A. Apolipoprotein serum levels related to metabolic syndrome in patients with schizophrenia. Heliyon 2019, 5, e02033. [Google Scholar] [CrossRef]
- Sakhalkar, V.S.; Rao, S.P.; Weedon, J.; Miller, S.T. Elevated plasma sVCAM-1 levels in children with sickle cell disease: Impact of chronic transfusion therapy. Am. J. Hematol. 2004, 76, 57–60. [Google Scholar] [CrossRef]
- Gonzalez-Candia, A.; Veliz, M.; Carrasco-Pozo, C.; Castillo, R.L.; Cárdenas, J.C.; Ebensperger, G.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Antenatal melatonin modulates an enhanced antioxidant/pro-oxidant ratio in pulmonary hypertensive newborn sheep. Redox Biol. 2019, 22, 101128. [Google Scholar] [CrossRef]
- Kaneva, A.M.; Potolitsyna, N.N.; Bojko, E.R.; Odland, J. The apolipoprotein B/apolipoprotein A-I ratio as a potential marker of plasma atherogenicity. Dis. Markers 2015, 2015, 591454. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I.; Aastveit, A.H.; Holme, I.; Furberg, C.D.; Sniderman, A.D. The apoB/apoA-I ratio is better than the cholesterol ratios to estimate the balance between plasma proatherogenic and antiatherogenic lipoproteins and to predict coronary risk. Clin. Chem. Lab. Med. 2004, 42, 1355–1363. [Google Scholar] [CrossRef]
- Dobiásová, M. AIP—Atherogenic index of plasma as a significant predictor of cardiovascular risk: From research to practice. Vnitr. Lek. 2006, 52, 64–71. [Google Scholar]
- Niroumand, S.; Khajedaluee, M.; Khadem-Rezaiyan, M.; Abrishami, M.; Juya, M.; Khodaee, G.; Dadgarmoghaddam, M. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med. J. Islam. Repub. Iran 2015, 29, 240. [Google Scholar]
- Marseille-Tremblay, C.; Ethier-Chiasson, M.; Forest, J.C.; Giguère, Y.; Masse, A.; Mounier, C.; Lafond, J. Impact of maternal circulating cholesterol and gestational diabetes mellitus on lipid metabolism in human term placenta. Mol. Reprod. Dev. 2008, 75, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liang, N.; Wang, H.; Gao, A.; Xiao, R.; Yu, H. Lipidomic profiles of maternal blood at the earlier stage of gestation and umbilical venous blood in response to supraphysiological hypercholesterolemia versus physiological hypercholesterolemia: An evidence of potential biomarkers and early intervention. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158587. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.D.; Yang, L.W.; Deng, D.Y.; Jiang, R.N.; Song, Z.K.; Zhou, L.T. The effects of brominated flame retardants (BFRs) on pro-atherosclerosis mechanisms. Ecotoxicol. Environ. Saf. 2023, 262, 115325. [Google Scholar] [CrossRef]
- Vuong, A.M.; Braun, J.M.; Sjödin, A.; Calafat, A.M.; Yolton, K.; Lanphear, B.P.; Chen, A. Exposure to endocrine disrupting chemicals (EDCs) and cardiometabolic indices during pregnancy: The HOME Study. Environ. Int. 2021, 156, 106747. [Google Scholar] [CrossRef]
- Kozlova, E.V.; Denys, M.E.; Benedum, J.; Valdez, M.C.; Enriquez, D.; Bishay, A.E.; Chinthirla, B.D.; Truong, E.; Krum, J.M.; DiPatrizio, N.V.; et al. Developmental exposure to indoor flame retardants and hypothalamic molecular signatures: Sex-dependent reprogramming of lipid homeostasis. Front. Endocrinol. 2022, 13, 997304. [Google Scholar] [CrossRef]
- Lorigo, M.; Cairrao, E. Fetoplacental vasculature as a model to study human cardiovascular endocrine disruption. Mol. Aspects Med. 2022, 87, 101054. [Google Scholar] [CrossRef]
- Kayden, H.J. The neurologic syndrome of vitamin E deficiency: A significant cause of ataxia. Neurology 1993, 43, 2167–2169. [Google Scholar] [CrossRef]
- Anwar, K.; Iqbal, J.; Hussain, M.M. Mechanisms involved in vitamin E transport by primary enterocytes and in vivo absorption. J. Lipid Res. 2007, 48, 2028–2038. [Google Scholar] [CrossRef]
- Anwar, K.; Kayden, H.J.; Hussain, M.M. Transport of vitamin E by differentiated Caco-2 cells. J. Lipid Res. 2006, 47, 1261–1273. [Google Scholar] [CrossRef]
- Besler, C.; Heinrich, K.; Rohrer, L.; Doerries, C.; Riwanto, M.; Shih, D.M.; Chroni, A.; Yonekawa, K.; Stein, S.; Schaefer, N.; et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Investig. 2011, 121, 2693–2708. [Google Scholar] [CrossRef]
- Iglesias, A.; Montelongo, A.; Herrera, E.; Lasunción, M.A. Changes in cholesteryl ester transfer protein activity during normal gestation and postpartum. Clin. Biochem. 1994, 27, 63–68. [Google Scholar] [CrossRef]
- Belo, L.; Caslake, M.; Santos-Silva, A.; Castro, E.M.; Pereira-Leite, L.; Quintanilha, A.; Rebelo, I. LDL size, total antioxidant status and oxidised LDL in normal human pregnancy: A longitudinal study. Atherosclerosis 2004, 177, 391–399. [Google Scholar] [CrossRef]
- Tian, L.; Wu, J.; Fu, M.; Xu, Y.; Jia, L. Relationship between apolipoprotein C-III concentrations and high-density lipoprotein subclass distribution. Metabolism 2009, 58, 668–674. [Google Scholar] [CrossRef]
- Tian, L.; Xu, Y.; Fu, M.; Jia, L.; Yang, Y. Influence of apolipoproteinCII concentrations on HDL subclass distribution. J. Atheroscler. Thromb. 2009, 16, 611–620. [Google Scholar] [CrossRef]
- Davidson, M.H.; Maki, K.C.; Bays, H.; Carter, R.; Ballantyne, C.M. Effects of prescription omega-3-acid ethyl esters on lipoprotein particle concentrations, apolipoproteins AI and CIII, and lipoprotein-associated phospholipase A(2) mass in statin-treated subjects with hypertriglyceridemia. J. Clin. Lipidol. 2009, 3, 332–340. [Google Scholar] [CrossRef]
- Cammisotto, V.; Baratta, F.; Simeone, P.G.; Barale, C.; Lupia, E.; Galardo, G.; Santilli, F.; Russo, I.; Pignatelli, P. Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Beyond Lipids: The Role in Oxidative Stress and Thrombosis. Antioxidants 2022, 11, 569. [Google Scholar] [CrossRef]
- Desideri, G.; Marinucci, M.C.; Tomassoni, G.; Masci, P.G.; Santucci, A.; Ferri, C. Vitamin E supplementation reduces plasma vascular cell adhesion molecule-1 and von Willebrand factor levels and increases nitric oxide concentrations in hypercholesterolemic patients. J. Clin. Endocrinol. Metab. 2002, 87, 2940–2945. [Google Scholar] [CrossRef]
- Lyall, F.; Greer, I.A.; Boswell, F.; Macara, L.M.; Walker, J.J.; Kingdom, J.C. The cell adhesion molecule, VCAM-1, is selectively elevated in serum in pre-eclampsia: Does this indicate the mechanism of leucocyte activation? Br. J. Obstet. Gynaecol. 1994, 101, 485–487. [Google Scholar] [CrossRef]
- Djurovic, S.; Schjetlein, R.; Wisløff, F.; Haugen, G.; Berg, K. Increased levels of intercellular adhesion molecules and vascular cell adhesion molecules in pre-eclampsia. Br. J. Obstet. Gynaecol. 1997, 104, 466–470. [Google Scholar] [CrossRef]
- Krauss, T.; Kuhn, W.; Lakoma, C.; Augustin, H.G. Circulating endothelial cell adhesion molecules as diagnostic markers for the early identification of pregnant women at risk for development of preeclampsia. Am. J. Obstet. Gynecol. 1997, 177, 443–449. [Google Scholar] [CrossRef]
- Hubel, C.A.; Lyall, F.; Weissfeld, L.; Gandley, R.E.; Roberts, J.M. Small low-density lipoproteins and vascular cell adhesion molecule-1 are increased in association with hyperlipidemia in preeclampsia. Metabolism 1998, 47, 1281–1288. [Google Scholar] [CrossRef]
- Szarka, A.; Rigó, J.; Lázár, L.; Beko, G.; Molvarec, A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010, 11, 59. [Google Scholar] [CrossRef]
- Coata, G.; Pennacchi, L.; Bini, V.; Liotta, L.; Di Renzo, G.C. Soluble adhesion molecules: Marker of pre-eclampsia and intrauterine growth restriction. J. Matern. Fetal Neonatal Med. 2002, 12, 28–34. [Google Scholar] [CrossRef]
- Millán, J.; Pintó, X.; Muñoz, A.; Zúñiga, M.; Rubiés-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernández-Mijares, A.; González-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar]
- Tamang, H.K.; Timilsina, U.; Singh, K.P.; Shrestha, S.; Raman, R.K.; Panta, P.; Karna, P.; Khadka, L.; Dahal, C. Apo B/Apo A-I Ratio is Statistically A Better Predictor of Cardiovascular Disease (CVD) than Conventional Lipid Profile: A Study from Kathmandu Valley, Nepal. J. Clin. Diagn. Res. 2014, 8, 34–36. [Google Scholar] [CrossRef]
- Cai, G.; Shi, G.; Xue, S.; Lu, W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine 2017, 96, e8058. [Google Scholar] [CrossRef]
- Timur, H.; Daglar, H.K.; Kara, O.; Kirbas, A.; Inal, H.A.; Turkmen, G.G.; Yilmaz, Z.; Elmas, B.; Uygur, D. A study of serum Apo A-1 and Apo B-100 levels in women with preeclampsia. Pregnancy Hypertens. 2016, 6, 121–125. [Google Scholar] [CrossRef]
- Piechota, W.; Staszewski, A. Reference ranges of lipids and apolipoproteins in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992, 45, 27–35. [Google Scholar] [CrossRef]



| Group | MPH (n = 34) | MSPH (n = 23) |
|---|---|---|
| Maternal variables | ||
| Weeks of gestation | 38.9 ± 0.9 (37–41) | 39.4 ± 0.8 (38–41) |
| Age (years) | 31.4 ± 4.8 (22–40) | 31.1 ± 5.8 (24–48) |
| Height (cm) | 160.8 ± 6.5 (150–173) | 160 ± 8.1 (148–180) |
| Weight (kg) | ||
| T1 | 61.7 ± 8.1 (48–81) | 59.6 ± 8.7 (47–82) |
| T2 | 67.7 ± 7.3 (54–87) | 65.5 ± 8 (50–82) |
| T3 | 71.9 ± 7.3 (61–86) | 70.1 ± 7.1 (55–85) |
| BMI (kg/m2) | ||
| T1 | 23.8 ± 2.5 (19.5–29.7) | 23.2 ± 2.4 (18.6–29) |
| T2 | 26.2 ± 2 (23.1–30.9) | 25.6 ± 2.4 (19.8–30.1) |
| T3 | 27.8 ± 2.1 (23.8–33) | 27.3 ± 2.3 (21.8–30.9) |
| Weight gain (kg) | 10.2 ± 3.1 (2.3–16) | 10.4 ± 3.6 (3–16) |
| Systolic arterial pressure (mm Hg) | ||
| T1 | 109.3 ± 9.7 (90–120) | 110.1 ± 9.5 (100–130) |
| T2 | 109.4 ± 9.1 (90–125) | 106.8 ± 9.7 (90–130) |
| T3 | 110.9 ± 8.4 (100–130) | 107 ± 9.3 (96–130) |
| Diastolic arterial pressure (mm Hg) | ||
| T1 | 67 ± 7.2 (50–80) | 68.8 ± 8 (60–80) |
| T2 | 67.2 ± 6.1 (60–80) | 64.1 ± 8.5 (40–80) |
| T3 | 69.5 ± 7.4 (53–80) | 67.9 ± 8.8 (55–90) |
| OGTT (mg/dL) | ||
| Basal glycaemia | 77.1 ± 8.5 (61–93) | 76.6 ± 6.4 (68–90) |
| Glycaemia 2 h after glucose | 103.6 ± 19.3 (71–135) | 101.5 ± 18.1 (65–129) |
| Parity | ||
| Primiparous | 11 (32.4%) | 8 (34.8%) |
| 1 | 18 (52.9%) | 11 (47.8%) |
| ≥2 | 5 (14.7%) | 4 (17.4%) |
| Lipid levels at delivery (mg/dL) | ||
| Total cholesterol | 232.7 ± 34.5 (168–280) | 318.9 ± 28.1 * (285–402) |
| HDL | 62.2 ± 12.8 (35–106) | 63.2 ± 15.9 (33–94) |
| LDL | 122.8 ± 31.5 (61–169) | 202.2 ± 26.3 * (157–273) |
| VLDL | 48 ± 12.4 (25–77) | 53.6 ± 13.1 (27–79) |
| Triglycerides | 239.8 ± 62.1 (124–385) | 267.4 ± 65.4 (133–393) |
| Newborn variables | ||
| Sex (female/male) | 16/18 | 10/13 |
| Birth weight (g) | 3375 ± 363.5 (2705–4230) | 3545 ± 387.9 (2750–4375) |
| Height (cm) | 49.2 ± 1.7 (46–52) | 49.8 ± 1.2 (48–52) |
| Ponderal index (g/cm3 × 100) | 2.8 ± 0.2 (2.4–3.3) | 2.9 ± 0.2 (2.5–3.2) |
| Cytokine | MPH (n = 15) | MSPH (n = 15) |
|---|---|---|
| IL-1β | 3.71 ± 0.67 | 3.66 ± 1.72 |
| IL-6 | 3.54 ± 1.07 | 3.99 ± 1.33 |
| IL-8 | 9.7 ± 5.24 | 10.1 ± 8.13 |
| IL-10 | 2.54 ± 0.46 | 2.92 ± 0.39 * |
| IL-12p70 | 1.5 ± 0.32 | 1.74 ± 0.27 * |
| TNF | 1.84 ± 0.53 | 1.81 ± 0.37 |
| Apolipoprotein | MPH (n = 10) | MSPH (n = 10) |
|---|---|---|
| ApoAI | 1.15 ± 0.38 | 1.05 ± 0.32 |
| ApoB | 1.67 ± 0.45 | 2.37 ± 0.58 * |
| ApoAII | 0.24 ± 0.04 | 0.27 ± 0.048 |
| ApoE | 0.05 ± 0.013 | 0.06 ± 0.014 |
| ApoCII | 0.15 ± 0.047 | 0.23 ± 0.062 * |
| ApoCIII | 0.49 ± 0.08 | 0.6 ± 0.1 * |
| ApoB/ApoAI | 1.43 ± 0.52 | 2.43 ± 0.93 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantin, C.; Morales, A.; Serra, R.; Illanes, S.E.; Leiva, A. Maternal Supraphysiological Hypercholesterolemia Is Accompanied by Shifts in the Composition and Anti-Atherogenic Functions of Maternal HDL along with Maternal Cardiovascular Risk Markers at Term of Pregnancy. Antioxidants 2023, 12, 1804. https://doi.org/10.3390/antiox12101804
Cantin C, Morales A, Serra R, Illanes SE, Leiva A. Maternal Supraphysiological Hypercholesterolemia Is Accompanied by Shifts in the Composition and Anti-Atherogenic Functions of Maternal HDL along with Maternal Cardiovascular Risk Markers at Term of Pregnancy. Antioxidants. 2023; 12(10):1804. https://doi.org/10.3390/antiox12101804
Chicago/Turabian StyleCantin, Claudette, Andrea Morales, Ramón Serra, Sebastián E. Illanes, and Andrea Leiva. 2023. "Maternal Supraphysiological Hypercholesterolemia Is Accompanied by Shifts in the Composition and Anti-Atherogenic Functions of Maternal HDL along with Maternal Cardiovascular Risk Markers at Term of Pregnancy" Antioxidants 12, no. 10: 1804. https://doi.org/10.3390/antiox12101804
APA StyleCantin, C., Morales, A., Serra, R., Illanes, S. E., & Leiva, A. (2023). Maternal Supraphysiological Hypercholesterolemia Is Accompanied by Shifts in the Composition and Anti-Atherogenic Functions of Maternal HDL along with Maternal Cardiovascular Risk Markers at Term of Pregnancy. Antioxidants, 12(10), 1804. https://doi.org/10.3390/antiox12101804