Role of 3-Mercaptopyruvate Sulfurtransferase (3-MST) in Physiology and Disease
Abstract
:1. Introduction
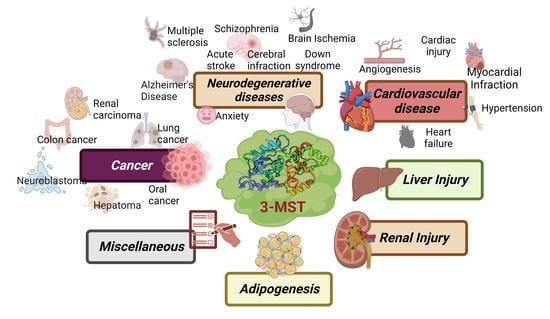
2. Role of 3-MST in Disease
2.1. Cancer
2.2. Cardiovascular Disorders
2.3. Neurological Disorders
2.4. Cyanide Toxicity
2.5. Miscellaneous
2.5.1. Obesity/Diabetes
2.5.2. Vasculature and Muscles
2.5.3. Osteoarthritis
2.5.4. Gastrointestinal System
2.5.5. Liver Injury
2.5.6. Microbes
3. Conclusions and Future Directions
| Model Used | Observation/Findings | |
|---|---|---|
| Cancer | ||
| Colon cancer | Mutant organoids of human intestinal epithelium [34], HCT116, HT29, and Lovo cells [36,52,125] 5-Flourouracil resistant HCT116 [37], CT26 murine carcinoma cells [39] Colon cancer tissues patients [52] | Upregulation of 3-MST/H2S pathway at early stage of cancer development [125]. H2S produced by 3-MST and other H2S-producing enzymes activated the CyR61 promoter by sulfhydration of Sp1 (CyR61 activator) [35]. Increased level of 3-MST also displayed cancer cell protective effect 1 [37,39,52]. Increased 3-MST expression contributed to generation of H2S in 5-FU-resistant cells [37] and regulated the CT26 cell migration, proliferation, and bioenergetics in CT26 cells [39]. Endogenous H2S produced by 3-MST promoted the epithelial-to-mesenchymal transition (EMT) by enhancing the ATP citrate lyse (ACLY) expression involved in Wnt-β-catenin pathways [36]. |
| Hepatocellular carcinoma (HCC) | Liver tissues of patients with HCC [53], HCC cell lines, LM3 xenografts mice model [116] | Increased level of 3-MST in epithelial cell adhesion molecule containing cancerous stem cells isolated from HCC patients [53]. Low tumor growth rate was observed in HCC xenograft mice overexpressing 3-MST [116]. 3-MST overexpression greatly reduced cell proliferation and growth by triggering G1-phase cell cycle arrest and controlling the AKT/FOXO3a/Rb pathway in HCC cells [116]. |
| Renal cancer | Tissue samples from human patients [56], T24 and UMUC3 urothelial cancer cell lines [38] | Differential expression of 3-MST and other H2S-producing enzymes observed irrespective of renal cancer metastasis, size, grade, and recurrence [56]. Low level of 3-MST was observed in urothelial cancerous cell line as compared to normal cells [38]. |
| Glioblastoma (GB) | GB patients derived cells (PDC), mice xenograft model of small hairpin RNA-induced knockdown 3-MST PDC and shControl PDS [55] | 3-MST knockdown in PDC using small hairpin RNA impaired cell’s motility, shape, and invasion ability, resulting in less tumor burden and higher survival observed in mice xenografted with 3-MST deleted PDC compared to shControl PDS [55]. 3-MST-mediated protein persulfidation required to protect the cells from hyperoxidation [55]. |
| Human neoplastic cancer cells | Astrocytoma U373 cells, neuroblastoma SHSY5Y cells, melanoma, melanoma WM35 cells, A375 from solid metastatic cancer [40,42,60] | High 3-MST expression was observed in all cell lines. 3-MST also showed better activity than CSE in these cell lines, thus it was also considered as a major protein involved in sulfane sulfur production. H2S produced from CSE induced proapoptotic effects on human melanoma by reducing the activation of ERK/pERK and Apk/pApk pathways and by inhibiting NF-kB mediated anti-apoptotic genes [60]. |
| Lung adeno-carcinoma | Human lung adenocarcinoma tissues, mice xenograft model of human lung cancer developed using A549 cells [46] | Combinatorial therapy of H2S-producing enzyme inhibitors and chemotherapeutic agents had a greater beneficial effect in lung cancer. Inhibition of 3-MST along with other H2S producing enzymes impaired mitochondrial bioenergetics and decreased mitochondrial DNA repair capacity [46]. |
| Oral cancer | Tumor biopsies from ACC patients [57], biopsies from Mucoepidermoid Carcinoma (MEC) patients [58], biopsies from Oral Squamous cell carcinoma (OSCC) patients [59] | Levels of 3-MST and other H2S producing enzymes were increased more in human ACC [57], MEC [58], and OSCC [59] than adjacent benign tissues. |
| Cardiovascular Disorders | ||
| Angiogenesis | bEnd3 cells and male Sprague-Dawley rats [62] | Increased H2S production in bEnd3 cells and plasma H2S levels in rats reduced 3-MST activity [62]. Hyperglycemia impaired 3-MP/3-MST/H2S pathway and mitochondrial function; proangiogenic effect of 3-MP in vitro was associated with the activation of Akt and Protein Kinase G (PKG) [62]. |
| Cardiac injury | Male Sprague-Dawley rats [126] | Lower 3-MST levels significantly increased NADPH Oxidase 4 (NOX4) and p67 protein expressions in cardiac injury [126]. H2S had cardio-protective effects via decreasing NADPH oxidase and ROS production. |
| Heart failure | Myocardial samples from patients and 3-MST knockout and wild-type mice subjected to acute heart failure [65], SD rats induced with Angiotensin-II and Left atrial appendage (LAA) tissue collected from rheumatic heart disease (RHD) patients [69] | Reduced 3-MST levels observed in failing patients [65]; reduced 3-MST and H2S levels in RHD patients [69]. Induction of heart failure in 3-MST KO mice led to poor exercise performance due to increased branched-chain amino acid accumulation in the myocardium, which was linked to decreased mitochondrial respiration, ATP synthesis, and exacerbated cardiac and vascular dysfunction [65]. Atrial Fibrillation reduced 3-MST expression and H2S level, increased ERS and atrial fibrosis, and promote left atrial dysfunction in SD rats [69]. |
| Hypertension | Male Wistar-Kyoto rats [70], blood samples collected from hypertensive patients and normotensive patients [72] | Lower expression and reduced 3-MST activity was observed in old hypertensive rats compared to young hypertensive [70]; erythrocyte and serum H2S levels were higher [72]; CBS and CSE levels were not detected in erythrocytes. Thus, 3-MST/H2S pathway is activated in hypertensive patients [72] |
| Myocardial infarction | Sepsis model was induced in Sprague-Dawley rats by cecal ligation and puncture (CLP) [66]; primary cultures of neonatal cardiomyocytes and adult male C57BL/6 mice [67] | Levels of 3-MST were reduced in the sepsis model [66]. Reduced plasma H2S levels corresponded with increased expression of endoplasmic reticulum stress marker proteins, including p-PERK, p-eIF2, IRE1α, ATF4, and CHOP [66]. Myocardial infarction surgery decreased 3-MST levels [67]. |
| Neurodegenerative Diseases | ||
| Acute stroke | Permanent occlusion of the left middle cerebral artery was induced in the SD rats [127] | Downregulation of 3-MST in both cortex and striatum [127]. |
| Alzheimer’s disease | Male APPswe/PS1dE9 AD mice and matched wild-type WT (C57B6) mice [83], SHSY-5Y cells, and APP/PS1 mice [82] | Reduced 3-MST expression [83]; reduced 3-MST activity and 3-MP levels [82] in APP/PS1 mice brain. Increased APP, BACE-1, and Aβ42 levels were observed in APP/PS1 mice compared to WT mice; however, NaSH treatment led to activation of Nrf2/ARE pathway [83]. Attenuation of neuroinflammation (TNFα, IL-6), elevated Aβ42 levels and oxidative stress in APP/PS1 mice by 3-MP prodrug, sulfanegen with restoration of cognitive impairment [82]. |
| Anxiety-like behaviors | 3-MST KO mice using C57BL/6 embryonic stem cells [20] | MST KO mice showed increased anxiety-like behavior and increased 5-hydroxyindoleacetic acid (5-HIAA) and 5-hydroxytryptamine (5-HT) levels [20]. |
| Down’s syndrome | Human dermal fibroblasts [73] | Increased 3-MST levels were found in human Down syndrome fibroblasts compared to controls [73]. Pharmacological suppression of 3-MST activity increased cell proliferation and mitochondrial electron transport and oxidative phosphorylation [73]. |
| Ischemia/reperfusion injury | PC-12 cells and male SD rats [78] | Betaine attenuates oxidative stress damage in vitro and I/R induced brain damage [78]. Increased inflammatory markers (IL-1β, IL-6 and TNFα), glutathione peroxidase 4 (Gpx4), superoxide dismutase 1 (Sod1), and 3-MST expression levels after I/R injury were reversed by betaine treatment [78]. |
| Multiple sclerosis (MS) | C57BL/6 mice femurs were used for the isolation of bone marrow cells and peripheral blood mononuclear cells (PBMC) obtained from MS patients [80] | Lower 3-MST function in PBMC from MS patients [80]. The expression of 3-MST and pro-inflammatory markers showed a significant inverse correlation [80]. |
| Hypoxia/oxygen-glucose deprivation | Primary brain vascular endothelial cells and SD rats [77] | OGD/R induced reduction in H2S and 3-MST levels in both ECs and mitochondria also enhanced oxidative stress. Cellular oxidative stress; reduction in mitochondrial potential and ATP levels/ATP synthase activity in hypoxia, which were ameliorated by 3-MP by inhibition of RhoA/ROCK pathway [77]. |
| Schizophrenia | B6 (C57BL6/NCrj) and C3H (C3H/HeNCrj) mice were used [128] | Proteomic analysis of brain in these strains showed elevated levels of 3-MST, H2S polysulfide-producing enzyme, and greater sulfide deposition in C3H than B6 mice [128]. 3-MST-Tg mice showed reduced ATP levels and decreased ATP-to-ADP ratio, and deficits in cytochrome c oxidase activity, compared to the non-Tg animals [128]. |
| Sleep deprivation | Adult male Wistar rats treated with 72 h sleep deprivation (SD) [74] | 3-MST levels in the hippocampus of SD-treated rats were reduced [74], triggering increase in autophagosomes, beclin-1 and LC3 II/LC3 I, and down-regulation of p62 [74]. |
| Subarachnoid hemorrhage | Human CSF samples and SD rats [81] | Increased 3-MST levels in human CSF samples after SAH [81], displaying correlations between increase in 3-MST and IL-6 [81]. |
| Traumatic brain injury | Adult male CD1 mice subjected to TBI [79] | Time-dependent increase in the levels of 3-MST reached peak after first day of injury and reached valley on the third day [79]. Upregulation of 3-MST in the brain cortex was associated with the neuronal autophagic protective effect after TBI, as 3-MST-expressing neurons partially displayed LC3 positive [79]. |
| Cyanide Toxicity | ||
| Cyanide toxicity | Human blood samples, HEK and A549 [129], Balb/co mice [85], pathogen-free white rabbits [92] | Three different polymorphisms with rare Tyr85 mutation were observed in human blood sample [129]. Individuals with non-sense mutation Tyr85 of 3-MST were more prone to develop cyanide-induced neurotoxicity [129]. 3-MST played a major role in cyanide detoxification in liver and kidney [85], and cyanide levels in erythrocytes, deoxyhemoglobin and oxyhemoglobin [92]. L-cysteine in the presence of 3-MST produced sulfane sulfur that was transferred by rhodanese to detoxify CN-forming SCN [85]. |
Funding
Acknowledgments
Conflicts of Interest
References
- Qabazard, B.; Li, L.; Gruber, J.; Peh, M.T.; Ng, L.F.; Kumar, S.D.; Rose, P.; Tan, C.H.; Dymock, B.W.; Wei, F.; et al. Hydrogen sulfide is an endogenous regulator of aging in Caenorhabditis elegans. Antioxid. Redox Signal. 2014, 20, 2621–2630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randi, E.B.; Casili, G.; Jacquemai, S.; Szabo, C. Selenium-Binding Protein 1 (SELENBP1) Supports Hydrogen Sulfide Biosynthesis and Adipogenesis. Antioxidants 2021, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Gotor, C.; Bassham, D.C.; Romero, L.C. Hydrogen Sulfide: From a Toxic Molecule to a Key Molecule of Cell Life. Antioxidants 2020, 9, 621. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide 2013, 35, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.K.; Banerjee, R. Detection of reaction intermediates during human cystathionine beta-synthase-monitored turnover and H2S production. J. Biol. Chem. 2012, 287, 43464–43471. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Jhee, K.H.; Kruger, W.D. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 2004, 279, 52082–52086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabil, O.; Zhou, Y.; Banerjee, R. Human cystathionine beta-synthase is a target for sumoylation. Biochemistry 2006, 45, 13528–13536. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Saha, S.; Giri, K.; Lanza, I.R.; Nair, K.S.; Jennings, N.B.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Basal, E.; Weaver, A.L.; et al. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS ONE 2013, 8, e79167. [Google Scholar] [CrossRef]
- Pan, L.L.; Liu, X.H.; Gong, Q.H.; Yang, H.B.; Zhu, Y.Z. Role of cystathionine gamma-lyase/hydrogen sulfide pathway in cardiovascular disease: A novel therapeutic strategy? Antioxid. Redox Signal. 2012, 17, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, N.; Kimura, H. Production of hydrogen sulfide from d-cysteine and its therapeutic potential. Front. Endocrinol. 2013, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Geng, B.; Yang, J.; Qi, Y.; Zhao, J.; Pang, Y.; Du, J.; Tang, C. H2S generated by heart in rat and its effects on cardiac function. Biochem. Biophys. Res. Commun. 2004, 313, 362–368. [Google Scholar] [CrossRef]
- Hosoki, R.; Matsuki, N.; Kimura, H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ndisang, J.F.; Wang, R. Modulation of endogenous production of H2S in rat tissues. Can. J. Physiol. Pharmacol. 2003, 81, 848–853. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H(2)S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Bordo, D.; Bork, P. The rhodanese/Cdc25 phosphatase superfamily. Sequence-structure-function relations. EMBO Rep. 2002, 3, 741–746. [Google Scholar] [CrossRef] [Green Version]
- Frasdorf, B.; Radon, C.; Leimkuhler, S. Characterization and interaction studies of two isoforms of the dual localized 3-mercaptopyruvate sulfurtransferase TUM1 from humans. J. Biol. Chem. 2014, 289, 34543–34556. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, N.; Okazaki, T.; Nishino, T. Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking similarity in active site amino acid sequence and the increase in the mercaptopyruvate sulfurtransferase activity of rhodanese by site-directed mutagenesis. J. Biol. Chem. 1995, 270, 16230–16235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahara, N.; Ito, T.; Kitamura, H.; Nishino, T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: Confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem. Cell Biol. 1998, 110, 243–250. [Google Scholar] [CrossRef]
- Nagahara, N.; Ito, T.; Minami, M. Mercaptopyruvate sulfurtransferase as a defense against cyanide toxication: Molecular properties and mode of detoxification. Histol. Histopathol. 1999, 14, 1277–1286. [Google Scholar] [CrossRef]
- Nagahara, N.; Nagano, M.; Ito, T.; Shimamura, K.; Akimoto, T.; Suzuki, H. Antioxidant enzyme, 3-mercaptopyruvate sulfurtransferase-knockout mice exhibit increased anxiety-like behaviors: A model for human mercaptolactate-cysteine disulfiduria. Sci. Rep. 2013, 3, 1986. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, N.; Katayama, A. Post-translational regulation of mercaptopyruvate sulfurtransferase via a low redox potential cysteine-sulfenate in the maintenance of redox homeostasis. J. Biol. Chem. 2005, 280, 34569–34576. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, N. Regulation of mercaptopyruvate sulfurtransferase activity via intrasubunit and intersubunit redox-sensing switches. Antioxid. Redox Signal. 2013, 19, 1792–1802. [Google Scholar] [CrossRef]
- Nagahara, N.; Yoshii, T.; Abe, Y.; Matsumura, T. Thioredoxin-dependent enzymatic activation of mercaptopyruvate sulfurtransferase. An intersubunit disulfide bond serves as a redox switch for activation. J. Biol. Chem. 2007, 282, 1561–1569. [Google Scholar] [CrossRef] [Green Version]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Ogasawara, Y.; Kimura, H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 2011, 439, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Shang, Q.; Yao, J.; Ji, Y. Hydrogen sulfide: A gaseous signaling molecule modulates tissue homeostasis: Implications in ophthalmic diseases. Cell Death Dis. 2019, 10, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabassum, R.; Jeong, N.Y. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int. J. Med. Sci. 2019, 16, 1386–1396. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Ding, L.; Xie, Z.Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Chang, P.; Yang, L.; Wang, Y.; Zhu, S.; Shan, H.; Zhang, M.; Tao, L. Neuroprotective effects of hydrogen sulfide on sodium azide-induced oxidative stress in PC12 cells. Int. J. Mol. Med. 2018, 41, 242–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Yamada, M.; Kimura, H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J. Biol. Chem. 2011, 286, 39379–39386. [Google Scholar] [CrossRef] [Green Version]
- Bora, P.; Manna, S.; Nair, M.A.; Sathe, R.R.M.; Singh, S.; Sreyas Adury, V.S.; Gupta, K.; Mukherjee, A.; Saini, D.K.; Kamat, S.S.; et al. Leveraging an enzyme/artificial substrate system to enhance cellular persulfides and mitigate neuroinflammation. Chem. Sci. 2021, 12, 12939–12949. [Google Scholar] [CrossRef]
- Libiad, M.; Yadav, P.K.; Vitvitsky, V.; Martinov, M.; Banerjee, R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem. 2014, 289, 30901–30910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishanina, T.V.; Libiad, M.; Banerjee, R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat. Chem. Biol. 2015, 11, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, N.; Nishino, T. Role of amino acid residues in the active site of rat liver mercaptopyruvate sulfurtransferase. CDNA cloning, overexpression, and site-directed mutagenesis. J. Biol. Chem. 1996, 271, 27395–27401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascencao, K.; Dilek, N.; Zuhra, K.; Modis, K.; Sato, T.; Szabo, C. Sequential Accumulation of ‘Driver’ Pathway Mutations Induces the Upregulation of Hydrogen-Sulfide-Producing Enzymes in Human Colonic Epithelial Cell Organoids. Antioxidants 2022, 11, 1823. [Google Scholar] [CrossRef] [PubMed]
- Ascencao, K.; Lheimeur, B.; Szabo, C. Regulation of CyR61 expression and release by 3-mercaptopyruvate sulfurtransferase in colon cancer cells. Redox Biol. 2022, 56, 102466. [Google Scholar] [CrossRef] [PubMed]
- Ascencao, K.; Dilek, N.; Augsburger, F.; Panagaki, T.; Zuhra, K.; Szabo, C. Pharmacological induction of mesenchymal-epithelial transition via inhibition of H2S biosynthesis and consequent suppression of ACLY activity in colon cancer cells. Pharmacol. Res. 2021, 165, 105393. [Google Scholar] [CrossRef]
- Untereiner, A.A.; Pavlidou, A.; Druzhyna, N.; Papapetropoulos, A.; Hellmich, M.R.; Szabo, C. Drug resistance induces the upregulation of H(2)S-producing enzymes in HCT116 colon cancer cells. Biochem. Pharmacol. 2018, 149, 174–185. [Google Scholar] [CrossRef]
- Panza, E.; Bello, I.; Smimmo, M.; Brancaleone, V.; Mitidieri, E.; Bucci, M.; Cirino, G.; Sorrentino, R.; D Emmanuele di Villa Bianca, R. Endogenous and exogenous hydrogen sulfide modulates urothelial bladder carcinoma development in human cell lines. Biomed. Pharmacother. 2022, 151, 113137. [Google Scholar] [CrossRef]
- Augsburger, F.; Randi, E.B.; Jendly, M.; Ascencao, K.; Dilek, N.; Szabo, C. Role of 3-Mercaptopyruvate Sulfurtransferase in the Regulation of Proliferation, Migration, and Bioenergetics in Murine Colon Cancer Cells. Biomolecules 2020, 10, 447. [Google Scholar] [CrossRef] [Green Version]
- Jurkowska, H.; Wrobel, M. Inhibition of Human Neuroblastoma Cell Proliferation by N-acetyl-L-cysteine as a Result of Increased Sulfane Sulfur Level. Anticancer Res. 2018, 38, 5109–5113. [Google Scholar] [CrossRef]
- Jurkowska, H.; Wrobel, M.; Kaczor-Kaminska, M.; Jasek-Gajda, E. A possible mechanism of inhibition of U87MG and SH-SY5Y cancer cell proliferation by diallyl trisulfide and other aspects of its activity. Amino Acids 2017, 49, 1855–1866. [Google Scholar] [CrossRef] [Green Version]
- Jurkowska, H.; Placha, W.; Nagahara, N.; Wrobel, M. The expression and activity of cystathionine-gamma-lyase and 3-mercaptopyruvate sulfurtransferase in human neoplastic cell lines. Amino Acids 2011, 41, 151–158. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A.; Akakura, S.; Sanokawa-Akakura, R.; Goodwin, S.; Tabibzadeh, S. Dedifferentiation of cancer cells following recovery from a potentially lethal damage is mediated by H2S-Nampt. Exp. Cell Res. 2015, 330, 135–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modis, K.; Coletta, C.; Erdelyi, K.; Papapetropoulos, A.; Szabo, C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013, 27, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.W.; Ye, M.J.; Yang, D.L.; Yu, M.P.; Zhou, C.F.; Shen, T. Hydrogen sulfide attenuates paraquat-induced epithelial-mesenchymal transition of human alveolar epithelial cells through regulating transforming growth factor-beta1/Smad2/3 signaling pathway. J. Appl. Toxicol. 2019, 39, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, B.; Marcatti, M.; Zatarain, J.R.; Druzhyna, N.; Wiktorowicz, J.E.; Nagy, P.; Hellmich, M.R.; Szabo, C. Inhibition of hydrogen sulfide biosynthesis sensitizes lung adenocarcinoma to chemotherapeutic drugs by inhibiting mitochondrial DNA repair and suppressing cellular bioenergetics. Sci. Rep. 2016, 6, 36125. [Google Scholar] [CrossRef] [Green Version]
- Breza, J., Jr.; Soltysova, A.; Hudecova, S.; Penesova, A.; Szadvari, I.; Babula, P.; Chovancova, B.; Lencesova, L.; Pos, O.; Breza, J.; et al. Endogenous H(2)S producing enzymes are involved in apoptosis induction in clear cell renal cell carcinoma. BMC Cancer 2018, 18, 591. [Google Scholar] [CrossRef]
- Gai, J.W.; Qin, W.; Liu, M.; Wang, H.F.; Zhang, M.; Li, M.; Zhou, W.H.; Ma, Q.T.; Liu, G.M.; Song, W.H.; et al. Expression profile of hydrogen sulfide and its synthases correlates with tumor stage and grade in urothelial cell carcinoma of bladder. Urol. Oncol. 2016, 34, 166.e15–166.e20. [Google Scholar] [CrossRef]
- Wahafu, W.; Gai, J.; Song, L.; Ping, H.; Wang, M.; Yang, F.; Niu, Y.; Xing, N. Increased H(2)S and its synthases in urothelial cell carcinoma of the bladder, and enhanced cisplatin-induced apoptosis following H(2)S inhibition in EJ cells. Oncol. Lett. 2018, 15, 8484–8490. [Google Scholar] [CrossRef]
- Wang, M.; Yan, J.; Cao, X.; Hua, P.; Li, Z. Hydrogen sulfide modulates epithelial-mesenchymal transition and angiogenesis in non-small cell lung cancer via HIF-1alpha activation. Biochem. Pharmacol. 2020, 172, 113775. [Google Scholar] [CrossRef]
- Bantzi, M.; Augsburger, F.; Loup, J.; Berset, Y.; Vasilakaki, S.; Myrianthopoulos, V.; Mikros, E.; Szabo, C.; Bochet, C.G. Novel Aryl-Substituted Pyrimidones as Inhibitors of 3-Mercaptopyruvate Sulfurtransferase with Antiproliferative Efficacy in Colon Cancer. J. Med. Chem. 2021, 64, 6221–6240. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.; Modis, K.; Toro, G.; Hellmich, M.R.; Szczesny, B.; Szabo, C. Role of endogenous and exogenous nitric oxide, carbon monoxide and hydrogen sulfide in HCT116 colon cancer cell proliferation. Biochem. Pharmacol. 2018, 149, 186–204. [Google Scholar] [CrossRef]
- Khosla, R.; Hemati, H.; Rastogi, A.; Ramakrishna, G.; Sarin, S.K.; Trehanpati, N. miR-26b-5p helps in EpCAM+cancer stem cells maintenance via HSC71/HSPA8 and augments malignant features in HCC. Liver Int. 2019, 39, 1692–1703. [Google Scholar] [CrossRef]
- Li, M.; Song, X.; Jin, Q.; Chen, Y.; Zhang, J.; Gao, J.; Cen, L.; Lin, Y.; Xu, C.; He, X.; et al. 3-Mercaptopyruvate sulfurtransferase represses tumour progression and predicts prognosis in hepatocellular carcinoma. Liver Int. 2022, 42, 1173–1184. [Google Scholar] [CrossRef]
- Saurty-Seerunghen, M.S.; Daubon, T.; Bellenger, L.; Delaunay, V.; Castro, G.; Guyon, J.; Rezk, A.; Fabrega, S.; Idbaih, A.; Almairac, F.; et al. Glioblastoma cell motility depends on enhanced oxidative stress coupled with mobilization of a sulfurtransferase. Cell Death Dis. 2022, 13, 913. [Google Scholar] [CrossRef]
- Sogutdelen, E.; Pacoli, K.; Juriasingani, S.; Akbari, M.; Gabril, M.; Sener, A. Patterns of Expression of H(2)S-Producing Enzyme in Human Renal Cell Carcinoma Specimens: Potential Avenue for Future Therapeutics. In Vivo 2020, 34, 2775–2781. [Google Scholar] [CrossRef]
- Dongsoo, K.; Chen, J.; Wei, E.; Ansari, J.; Meram, A.; Patel, S.; Ghali, G.; Kevil, C.; Shackelford, R.E. Hydrogen Sulfide and Hydrogen Sulfide-Synthesizing Enzymes Are Altered in a Case of Oral Adenoid Cystic Carcinoma. Case Rep. Oncol. 2018, 11, 585–590. [Google Scholar] [CrossRef]
- Kim, D.; Chen, J.; Meram, A.; Patel, S.; Wei, E.; Ansari, J.; Ghali, G.; Kevil, C.; Shackelford, R.E. Hydrogen Sulfide-Synthesizing Enzymes Are Altered in a Case of Oral Cavity Mucoepidermoid Carcinoma. Case Rep. Oncol. 2018, 11, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Meram, A.T.; Chen, J.; Patel, S.; Kim, D.D.; Shirley, B.; Covello, P.; Coppola, D.; Wei, E.X.; Ghali, G.; Kevil, C.G.; et al. Hydrogen Sulfide Is Increased in Oral Squamous Cell Carcinoma Compared to Adjacent Benign Oral Mucosae. Anticancer Res. 2018, 38, 3843–3852. [Google Scholar] [CrossRef] [Green Version]
- Panza, E.; De Cicco, P.; Armogida, C.; Scognamiglio, G.; Gigantino, V.; Botti, G.; Germano, D.; Napolitano, M.; Papapetropoulos, A.; Bucci, M.; et al. Role of the cystathionine gamma lyase/hydrogen sulfide pathway in human melanoma progression. Pigment Cell Melanoma Res. 2015, 28, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi Govar, A.; Toro, G.; Szaniszlo, P.; Pavlidou, A.; Bibli, S.I.; Thanki, K.; Resto, V.A.; Chao, C.; Hellmich, M.R.; Szabo, C.; et al. 3-Mercaptopyruvate sulfurtransferase supports endothelial cell angiogenesis and bioenergetics. Br. J. Pharmacol. 2020, 177, 866–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coletta, C.; Modis, K.; Szczesny, B.; Brunyanszki, A.; Olah, G.; Rios, E.C.; Yanagi, K.; Ahmad, A.; Papapetropoulos, A.; Szabo, C. Regulation of Vascular Tone, Angiogenesis and Cellular Bioenergetics by the 3-Mercaptopyruvate Sulfurtransferase/H2S Pathway: Functional Impairment by Hyperglycemia and Restoration by DL-alpha-Lipoic Acid. Mol. Med. 2015, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Strutynska, N.; Strutynskyi, R.; Mys, L.; Luchkova, A.; Korkach, Y.; Goshovska, Y.; Chorna, S.; Sagach, V. Exercise restores endogenous H(2) S synthesis and mitochondrial function in the heart of old rats. Eur. J. Clin. Invest. 2022, 52, e13829. [Google Scholar] [CrossRef] [PubMed]
- Peleli, M.; Bibli, S.I.; Li, Z.; Chatzianastasiou, A.; Varela, A.; Katsouda, A.; Zukunft, S.; Bucci, M.; Vellecco, V.; Davos, C.H.; et al. Cardiovascular phenotype of mice lacking 3-mercaptopyruvate sulfurtransferase. Biochem. Pharmacol. 2020, 176, 113833. [Google Scholar] [CrossRef]
- Li, Z.; Xia, H.; Sharp, T.E., 3rd; LaPenna, K.B.; Elrod, J.W.; Casin, K.M.; Liu, K.; Calvert, J.W.; Chau, V.Q.; Salloum, F.N.; et al. Mitochondrial H(2)S Regulates BCAA Catabolism in Heart Failure. Circ. Res. 2022, 131, 222–235. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Cao, G.D.; Guo, L.C.; Cheng, Q.H. Hydrogen Sulfide Ameliorates Myocardial Injury Caused by Sepsis Through Suppressing ROS-Mediated Endoplasmic Reticulum Stress. Sichuan Da Xue Xue Bao Yi Xue Ban 2022, 53, 798–804. [Google Scholar] [CrossRef]
- Li, N.; Wang, M.J.; Jin, S.; Bai, Y.D.; Hou, C.L.; Ma, F.F.; Li, X.H.; Zhu, Y.C. The H2S Donor NaHS Changes the Expression Pattern of H2S-Producing Enzymes after Myocardial Infarction. Oxidative Med. Cell. Longev. 2016, 2016, 6492469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, M.M.; Kim, D.H.; Jandu, S.; Bergman, Y.; Tan, S.; Wang, H.; Pandey, D.R.; Abraham, T.P.; Shoukas, A.A.; Berkowitz, D.E.; et al. MPST but not CSE is the primary regulator of hydrogen sulfide production and function in the coronary artery. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H71–H79. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.J.; Wang, X.H.; Liu, Y.; Zhang, T.Q.; Chen, Z.R.; Zhang, C.; Tang, Z.H.; Qu, S.L.; Tang, H.F.; Jiang, Z.S. Hydrogen Sulfide Ameliorates Angiotensin II-Induced Atrial Fibrosis Progression to Atrial Fibrillation through Inhibition of the Warburg Effect and Endoplasmic Reticulum Stress. Front. Pharmacol. 2021, 12, 690371. [Google Scholar] [CrossRef]
- Szlezak, D.; Hutsch, T.; Ufnal, M.; Wrobel, M. Heart and kidney H(2)S production is reduced in hypertensive and older rats. Biochimie 2022, 199, 130–138. [Google Scholar] [CrossRef]
- Szlezak, D.; Bronowicka-Adamska, P.; Hutsch, T.; Ufnal, M.; Wrobel, M. Hypertension and Aging Affect Liver Sulfur Metabolism in Rats. Cells 2021, 10, 1238. [Google Scholar] [CrossRef]
- Zheng, M.; Zeng, Q.; Shi, X.Q.; Zhao, J.; Tang, C.S.; Sun, N.L.; Geng, B. Erythrocytic or serum hydrogen sulfide association with hypertension development in untreated essential hypertension. Chin. Med. J. 2011, 124, 3693–3701. [Google Scholar] [PubMed]
- Panagaki, T.; Randi, E.B.; Szabo, C. Role of 3-Mercaptopyruvate Sulfurtransferase in the Regulation of Proliferation and Cellular Bioenergetics in Human Down Syndrome Fibroblasts. Biomolecules 2020, 10, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.Q.; Jiang, L.; Lan, F.; Wei, H.J.; Xie, M.; Zou, W.; Zhang, P.; Wang, C.Y.; Xie, Y.R.; Tang, X.Q. Inhibited Endogenous H(2)S Generation and Excessive Autophagy in Hippocampus Contribute to Sleep Deprivation-Induced Cognitive Impairment. Front. Psychol. 2019, 10, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Xu, Z.; Yao, D.; Liu, X.; Liu, W.; Wang, N.; Li, X.; Diao, Y.; Zhang, Y.; Zhao, Q. An integrated multi-omics approach revealed the regulation of melatonin on age-dependent mitochondrial function impair and lipid dyshomeostasis in mice hippocampus. Pharmacol. Res. 2022, 179, 106210. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, C.; Manuel, M.L.; Yuan, S.; Kevil, C.G.; McCarter, K.D.; Lu, W.; Sun, H. Role of hydrogen sulfide in early blood-brain barrier disruption following transient focal cerebral ischemia. PLoS ONE 2015, 10, e0117982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, S.; Wen, J.Y.; Chen, Z.W. 3-Mercaptopyruvate sulfurtransferase/hydrogen sulfide protects cerebral endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury via mitoprotection and inhibition of the RhoA/ROCK pathway. Am. J. Physiol. Cell Physiol. 2020, 319, C720–C733. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qu, M.; Wang, N.; Wang, L.; Fan, G.; Yang, C. Betaine protects rats against ischemia/reperfusion injury-induced brain damage. J. Neurophysiol. 2022, 127, 444–451. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, H.; Chang, P.; Ma, L.; Chu, Y.; Shen, X.; Wu, Q.; Wang, Z.; Luo, C.; Wang, T.; et al. Upregulation of 3-MST Relates to Neuronal Autophagy After Traumatic Brain Injury in Mice. Cell Mol. Neurobiol. 2017, 37, 291–302. [Google Scholar] [CrossRef]
- Lazarevic, M.; Battaglia, G.; Jevtic, B.; Dedovic, N.; Bruno, V.; Cavalli, E.; Miljkovic, D.; Nicoletti, F.; Momcilovic, M.; Fagone, P. Upregulation of Tolerogenic Pathways by the Hydrogen Sulfide Donor GYY4137 and Impaired Expression of H(2)S-Producing Enzymes in Multiple Sclerosis. Antioxidants 2020, 9, 608. [Google Scholar] [CrossRef]
- Han, M.; Liu, D.; Qiu, J.; Yuan, H.; Hu, Q.; Xue, H.; Li, T.; Ma, W.; Zhang, Q.; Li, G.; et al. Evaluation of H(2)S-producing enzymes in cerebrospinal fluid and its relationship with interleukin-6 and neurologic deficits in subarachnoid hemorrhage. Biomed. Pharmacother. 2020, 123, 109722. [Google Scholar] [CrossRef]
- Rao, S.P.; Xie, W.; Christopher Kwon, Y.I.; Juckel, N.; Xie, J.; Dronamraju, V.R.; Vince, R.; Lee, M.K.; More, S.S. Sulfanegen stimulates 3-mercaptopyruvate sulfurtransferase activity and ameliorates Alzheimer’s disease pathology and oxidative stress in vivo. Redox Biol. 2022, 57, 102484. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.; Liu, H.; Yin, C.; Li, X.; Gong, Q. Hydrogen sulfide ameliorates learning memory impairment in APP/PS1 transgenic mice: A novel mechanism mediated by the activation of Nrf2. Pharmacol. Biochem. Behav. 2016, 150–151, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rao, P.; Bhattacharya, R. Dose and time-dependent effects of cyanide on thiosulfate sulfurtransferase, 3-mercaptopyruvate sulfurtransferase, and cystathionine lambda-lyase activities. J. Biochem. Mol. Toxicol. 2013, 27, 499–507. [Google Scholar] [CrossRef]
- Wrobel, M.; Jurkowska, H.; Sliwa, L.; Srebro, Z. Sulfurtransferases and cyanide detoxification in mouse liver, kidney, and brain. Toxicol. Mech. Methods 2004, 14, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Bilska, A.; Ksiazek, L.; Srebro, Z.; Wlodek, L. Allyl disulfide as donor and cyanide as acceptor of sulfane sulfur in the mouse tissues. Pharmacol. Rep. 2005, 57, 212–218. [Google Scholar] [PubMed]
- Wing, D.A.; Baskin, S.I. Modifiers of mercaptopyruvate sulfurtransferase catalyzed conversion of cyanide to thiocyanate in vitro. J. Biochem. Toxicol. 1992, 7, 65–72. [Google Scholar] [CrossRef]
- Wing, D.A.; Patel, H.C.; Baskin, S.I. The effect of picrylsulphonic acid on In vitro conversion of cyanide to thiocyanate by 3-mercaptopyruvate sulphurtransferase and rhodanese. Toxicol. In Vitro 1992, 6, 597–603. [Google Scholar] [CrossRef]
- Iciek, M.; Bilska, A.; Lorenc-Koci, E.; Wlodek, L.B.; Sokolowska, M.M. The effect of uremic toxin cyanate (OCN-) on anaerobic sulfur metabolism and prooxidative processes in the rat kidney: A protective role of lipoate. Hum. Exp. Toxicol. 2011, 30, 1601–1608. [Google Scholar] [CrossRef]
- Patterson, S.E.; Moeller, B.; Nagasawa, H.T.; Vince, R.; Crankshaw, D.L.; Briggs, J.; Stutelberg, M.W.; Vinnakota, C.V.; Logue, B.A. Development of sulfanegen for mass cyanide casualties. Ann. N. Y. Acad. Sci. 2016, 1374, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Patterson, S.E.; Monteil, A.R.; Cohen, J.F.; Crankshaw, D.L.; Vince, R.; Nagasawa, H.T. Cyanide antidotes for mass casualties: Water-soluble salts of the dithiane (sulfanegen) from 3-mercaptopyruvate for intramuscular administration. J. Med. Chem. 2013, 56, 1346–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, M.; Kim, J.G.; Lee, J.; Mahon, S.B.; Lemor, D.; Ahdout, R.; Boss, G.R.; Blackledge, W.; Jann, L.; Nagasawa, H.T.; et al. Sulfanegen sodium treatment in a rabbit model of sub-lethal cyanide toxicity. Toxicol. Appl. Pharmacol. 2010, 248, 269–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.X.; Wang, G.; Wu, W.; Gao, L.; Tan, Q.Y.; Wang, J. Hydrogen Sulfide Inhibits High Glucose-Induced sFlt-1 Production via Decreasing ADAM17 Expression in 3T3-L1 Adipocytes. Int. J. Endocrinol. 2017, 2017, 9501792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casili, G.; Randi, E.; Panagaki, T.; Zuhra, K.; Petrosino, M.; Szabo, C. Inhibition of the 3-mercaptopyruvate sulfurtransferase-hydrogen sulfide system promotes cellular lipid accumulation. Geroscience 2022, 44, 2271–2289. [Google Scholar] [CrossRef]
- Comas, F.; Latorre, J.; Ortega, F.; Arnoriaga Rodriguez, M.; Kern, M.; Lluch, A.; Ricart, W.; Bluher, M.; Gotor, C.; Romero, L.C.; et al. Activation of Endogenous H(2)S Biosynthesis or Supplementation with Exogenous H(2)S Enhances Adipose Tissue Adipogenesis and Preserves Adipocyte Physiology in Humans. Antioxid. Redox Signal. 2021, 35, 319–340. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Hydrogen sulfide regulates circadian-clock genes in C(2)C(12) myotubes and the muscle of high-fat-diet-fed mice. Arch. Biochem. Biophys. 2019, 672, 108054. [Google Scholar] [CrossRef]
- Peh, M.T.; Anwar, A.B.; Ng, D.S.; Atan, M.S.; Kumar, S.D.; Moore, P.K. Effect of feeding a high fat diet on hydrogen sulfide (H2S) metabolism in the mouse. Nitric Oxide 2014, 41, 138–145. [Google Scholar] [CrossRef]
- Katsouda, A.; Valakos, D.; Dionellis, V.S.; Bibli, S.I.; Akoumianakis, I.; Karaliota, S.; Zuhra, K.; Fleming, I.; Nagahara, N.; Havaki, S.; et al. MPST sulfurtransferase maintains mitochondrial protein import and cellular bioenergetics to attenuate obesity. J. Exp. Med. 2022, 219, 20211894. [Google Scholar] [CrossRef]
- Katsouda, A.; Szabo, C.; Papapetropoulos, A. Reduced adipose tissue H(2)S in obesity. Pharmacol. Res. 2018, 128, 190–199. [Google Scholar] [CrossRef]
- Kutz, J.L.; Greaney, J.L.; Santhanam, L.; Alexander, L.M. Evidence for a functional vasodilatatory role for hydrogen sulphide in the human cutaneous microvasculature. J. Physiol. 2015, 593, 2121–2129. [Google Scholar] [CrossRef] [Green Version]
- Madden, J.A.; Ahlf, S.B.; Dantuma, M.W.; Olson, K.R.; Roerig, D.L. Precursors and inhibitors of hydrogen sulfide synthesis affect acute hypoxic pulmonary vasoconstriction in the intact lung. J. Appl. Physiol. 2012, 112, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, R.; Provitera, L.; Cavallaro, G.; Lattuada, D.; Ercoli, G.; Mosca, F.; Villamor, E. Vasomotor effects of hydrogen sulfide in human umbilical vessels. J. Physiol. Pharmacol. 2017, 68, 737–747. [Google Scholar]
- Zhang, Y.; Yang, J.; Wang, T.; Wang, S.G.; Liu, J.H.; Yin, C.P.; Ye, Z.Q. Decreased Endogenous Hydrogen Sulfide Generation in Penile Tissues of Diabetic Rats with Erectile Dysfunction. J. Sex. Med. 2016, 13, 350–360. [Google Scholar] [CrossRef]
- Gai, J.W.; Wahafu, W.; Guo, H.; Liu, M.; Wang, X.C.; Xiao, Y.X.; Zhang, L.; Xin, Z.C.; Jin, J. Further evidence of endogenous hydrogen sulphide as a mediator of relaxation in human and rat bladder. Asian J. Androl. 2013, 15, 692–696. [Google Scholar] [CrossRef] [Green Version]
- Rashid, S.; Heer, J.K.; Garle, M.J.; Alexander, S.P.; Roberts, R.E. Hydrogen sulphide-induced relaxation of porcine peripheral bronchioles. Br. J. Pharmacol. 2013, 168, 1902–1910. [Google Scholar] [CrossRef] [Green Version]
- Yetik-Anacak, G.; Dikmen, A.; Coletta, C.; Mitidieri, E.; Dereli, M.; Donnarumma, E.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R. Hydrogen sulfide compensates nitric oxide deficiency in murine corpus cavernosum. Pharmacol. Res. 2016, 113, 38–43. [Google Scholar] [CrossRef]
- Nasi, S.; Ehirchiou, D.; Chatzianastasiou, A.; Nagahara, N.; Papapetropoulos, A.; Bertrand, J.; Cirino, G.; So, A.; Busso, N. The protective role of the 3-mercaptopyruvate sulfurtransferase (3-MST)-hydrogen sulfide (H(2)S) pathway against experimental osteoarthritis. Arthritis Res. Ther. 2020, 22, 49. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.W.; Zhang, Y.; Cheng, Y.Z.; Fan, X.S.; Deng, X.; Peng, H.Y. The expression of endogenous hydrogen sulfide signal during distraction osteogenesis in a rabbit model. Int. J. Oral. Maxillofac. Surg. 2018, 47, 262–267. [Google Scholar] [CrossRef]
- Bronowicka-Adamska, P.; Hutsch, T.; Gawrys-Kopczynska, M.; Maksymiuk, K.; Wrobel, M. Hydrogen sulfide formation in experimental model of acute pancreatitis. Acta Biochim. Pol. 2019, 66, 611–618. [Google Scholar] [CrossRef]
- Nagahara, N. Multiple role of 3-mercaptopyruvate sulfurtransferase: Antioxidative function, H(2) S and polysulfide production and possible SO(x) production. Br. J. Pharmacol. 2018, 175, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Magierowski, M.; Magierowska, K.; Hubalewska-Mazgaj, M.; Adamski, J.; Bakalarz, D.; Sliwowski, Z.; Pajdo, R.; Kwiecien, S.; Brzozowski, T. Interaction between endogenous carbon monoxide and hydrogen sulfide in the mechanism of gastroprotection against acute aspirin-induced gastric damage. Pharmacol. Res. 2016, 114, 235–250. [Google Scholar] [CrossRef]
- Magierowski, M.; Magierowska, K.; Szmyd, J.; Surmiak, M.; Sliwowski, Z.; Kwiecien, S.; Brzozowski, T. Hydrogen Sulfide and Carbon Monoxide Protect Gastric Mucosa Compromised by Mild Stress Against Alendronate Injury. Dig. Dis. Sci. 2016, 61, 3176–3189. [Google Scholar] [CrossRef] [Green Version]
- Bronowicka-Adamska, P.; Wrobel, M.; Magierowski, M.; Magierowska, K.; Kwiecien, S.; Brzozowski, T. Hydrogen Sulphide Production in Healthy and Ulcerated Gastric Mucosa of Rats. Molecules 2017, 22, 530. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cen, L.; Zhang, X.; Tang, C.; Chen, Y.; Zhang, Y.; Yu, M.; Lu, C.; Li, M.; Li, S.; et al. MPST deficiency promotes intestinal epithelial cell apoptosis and aggravates inflammatory bowel disease via AKT. Redox Biol. 2022, 56, 102469. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, S.; Teng, X.; Hu, Z.; Zhang, Z.; Qiu, X.; Tian, D.; Wu, Y. Hydrogen Sulfide Attenuates LPS-Induced Acute Kidney Injury by Inhibiting Inflammation and Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 6717212. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xu, C.; Shi, J.; Ding, J.; Wan, X.; Chen, D.; Gao, J.; Li, C.; Zhang, J.; Lin, Y.; et al. Fatty acids promote fatty liver disease via the dysregulation of 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Gut 2018, 67, 2169–2180. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, J.; Gu, Q. Exercise restores bioavailability of hydrogen sulfide and promotes autophagy influx in livers of mice fed with high-fat diet. Can. J. Physiol. Pharmacol. 2017, 95, 667–674. [Google Scholar] [CrossRef]
- Si, M.; Chen, C.; Zhong, J.; Li, X.; Liu, Y.; Su, T.; Yang, G. MsrR is a thiol-based oxidation-sensing regulator of the XRE family that modulates C. glutamicum oxidative stress resistance. Microb Cell Fact. 2020, 19, 189. [Google Scholar] [CrossRef]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A universal defense against antibiotics in bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Lacourciere, G.M.; Ishii, K.; Stadtman, T.C. Characterization of potential selenium-binding proteins in the selenophosphate synthetase system. Proc. Natl. Acad. Sci. USA 2005, 102, 1012–1016. [Google Scholar] [CrossRef] [Green Version]
- Wenzhong, W.; Tong, Z.; Hongjin, L.; Ying, C.; Jun, X. Role of Hydrogen Sulfide on Autophagy in Liver Injuries Induced by Selenium Deficiency in Chickens. Biol. Trace Elem. Res. 2017, 175, 194–203. [Google Scholar] [CrossRef]
- Zheng, S.F.; Bao, R.K.; Zhang, Q.J.; Wang, S.C.; Lin, H.J. Endogenous Hydrogen Sulfide Promotes Apoptosis via Mitochondrial Pathways in the Livers of Broilers with Selenium Deficiency Exudative Diathesis Disease. Biol. Trace Elem. Res. 2018, 186, 249–257. [Google Scholar] [CrossRef]
- Sen, U.; Sathnur, P.B.; Kundu, S.; Givvimani, S.; Coley, D.M.; Mishra, P.K.; Qipshidze, N.; Tyagi, N.; Metreveli, N.; Tyagi, S.C. Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. Am. J. Physiol. Cell Physiol. 2012, 303, C41–C51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanaoka, K.; Sasakura, K.; Suwanai, Y.; Toma-Fukai, S.; Shimamoto, K.; Takano, Y.; Shibuya, N.; Terai, T.; Komatsu, T.; Ueno, T.; et al. Discovery and Mechanistic Characterization of Selective Inhibitors of H(2)S-producing Enzyme: 3-Mercaptopyruvate Sulfurtransferase (3MST) Targeting Active-site Cysteine Persulfide. Sci. Rep. 2017, 7, 40227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascencao, K.; Szabo, C. Emerging roles of cystathionine beta-synthase in various forms of cancer. Redox Biol. 2022, 53, 102331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, S.; Teng, X.; Duan, X.; Chen, Y.; Wu, Y. Hydrogen sulfide attenuates cardiac injury in takotsubo cardiomyopathy by alleviating oxidative stress. Nitric Oxide 2017, 67, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chan, S.J.; Ng, Y.K.; Wong, P.T. Brain 3-Mercaptopyruvate Sulfurtransferase (3MST): Cellular Localization and Downregulation after Acute Stroke. PLoS ONE 2013, 8, e67322. [Google Scholar] [CrossRef] [Green Version]
- Ide, M.; Ohnishi, T.; Toyoshima, M.; Balan, S.; Maekawa, M.; Shimamoto-Mitsuyama, C.; Iwayama, Y.; Ohba, H.; Watanabe, A.; Ishii, T.; et al. Excess hydrogen sulfide and polysulfides production underlies a schizophrenia pathophysiology. EMBO Mol. Med. 2019, 11, e10695. [Google Scholar] [CrossRef]
- Billaut-Laden, I.; Rat, E.; Allorge, D.; Crunelle-Thibaut, A.; Cauffiez, C.; Chevalier, D.; Lo-Guidice, J.M.; Broly, F. Evidence for a functional genetic polymorphism of the human mercaptopyruvate sulfurtransferase (MPST), a cyanide detoxification enzyme. Toxicol. Lett. 2006, 165, 101–111. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.P.; Dobariya, P.; Bellamkonda, H.; More, S.S. Role of 3-Mercaptopyruvate Sulfurtransferase (3-MST) in Physiology and Disease. Antioxidants 2023, 12, 603. https://doi.org/10.3390/antiox12030603
Rao SP, Dobariya P, Bellamkonda H, More SS. Role of 3-Mercaptopyruvate Sulfurtransferase (3-MST) in Physiology and Disease. Antioxidants. 2023; 12(3):603. https://doi.org/10.3390/antiox12030603
Chicago/Turabian StyleRao, Swetha Pavani, Prakashkumar Dobariya, Harshini Bellamkonda, and Swati S. More. 2023. "Role of 3-Mercaptopyruvate Sulfurtransferase (3-MST) in Physiology and Disease" Antioxidants 12, no. 3: 603. https://doi.org/10.3390/antiox12030603
APA StyleRao, S. P., Dobariya, P., Bellamkonda, H., & More, S. S. (2023). Role of 3-Mercaptopyruvate Sulfurtransferase (3-MST) in Physiology and Disease. Antioxidants, 12(3), 603. https://doi.org/10.3390/antiox12030603