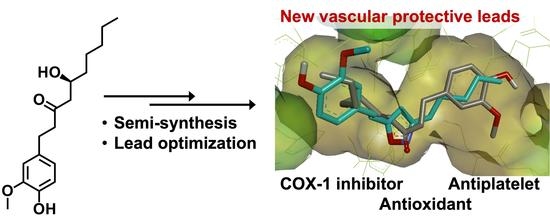
Preparation and Evaluation of 6-Gingerol Derivatives as Novel Antioxidants and Antiplatelet Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Isolation and Semi-synthesis
2.2.1. Purification of 6-gingerol ((S)-5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)decan-3-one) (1)
2.2.2. Synthesis of 1-(4-hydroxy-3-methoxyphenyl)decan-3-one oxime (4)
2.2.3. Synthesis of (3R,5S)-1-(4-hydroxy-3-methoxyphenyl)decane-3,5-diol (5) and (3S,5S)-1-(4-hydroxy-3-methoxy phenyl)decane-3,5-diol (6)
2.2.4. Synthesis of (E)-1-(4-hydroxyphenyl)dec-1-ene-3,5-dione (12)
2.2.5. Synthesis of 2-methoxy-4-(2-(3-pentyl-1H-pyrazol-5-yl)ethyl)phenol (14), 4-(2-(3-pentyl-1H-pyrazol-5-yl)ethyl)phenol (15), and (E)-4-(2-(3-pentyl-1H-pyrazol-5-yl)vinyl)phenol (16)
2.2.6. Synthesis of (E)-2-methoxy-4-(2-(3-pentylisoxazol-5-yl)vinyl)phenol (17) and (E)-4-(2-(3-pentylisoxazol-5-yl)vinyl)phenol (18)
2.2.7. Synthesis of N-heptyl-3-(4-hydroxyphenyl) propenamide (22)
2.3. Biological Activity
2.3.1. Antiplatelet Activity
2.3.2. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Scavenging Activity
2.3.3. Oxygen Radical Absorbance Capacity (ORAC)
2.3.4. Xanthine Oxidase Inhibitory Activity
2.3.5. Peroxynitrite Scavenging Activity
2.3.6. COX-1 Inhibitory Activity
2.3.7. Physicochemical Character and Blood–brain Barrier Specific Permeability
2.3.8. Molecular Docking
3. Results and Discussion
3.1. Chemistry
3.2. Predicted and Experimental Physicochemical and BBB Penetration-Related Characterisation
3.3. Antiplatelet Aggregation and COX-1 Inhibition Activity of the Compounds
3.4. Molecular Docking
3.5. Antioxidant Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yahyazadeh, R.; Rahimi, V.B.; Yahyazadeh, A.; Mohajeri, S.A.; Askari, V.R. Promising effects of gingerol against toxins: A review article. Biofactors 2021, 47, 885–913. [Google Scholar] [CrossRef]
- Fakhri, S.; Patra, J.K.; Das, S.K.; Das, G.; Majnooni, M.B.; Farzaei, M.H. Ginger and Heart Health: From Mechanisms to Therapeutics. Curr. Mol. Pharmacol. 2020, 14, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Roudsari, N.M.; Lashgari, N.; Momtaz, S.; Roufogalis, B.; Abdolghaffari, A.H.; Sahebkar, A. Ginger: A complementary approach for management of cardiovascular diseases. Biofactors 2021, 47, 933–951. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Saxena, R.; Rida, P.C.G.; Kucuk, O.; Aneja, R. Ginger augmented chemotherapy: A novel multitarget nontoxic approach for cancer management. Mol. Nutr. Food Res. 2016, 60, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Sp, N.; Kang, D.; Lee, J.-M.; Bae, S.; Jang, K.-J. Potential Antitumor Effects of 6-Gingerol in p53-Dependent Mitochondrial Apoptosis and Inhibition of Tumor Sphere Formation in Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 4660. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Nadeem, M.S.; Afzal, O.; Alzarea, S.I.; Altamimi, A.S.A.; Almalki, W.H.; Mubeen, B.; Iftikhar, S.; Shah, L.; Kazmi, I. Gingerol, a Natural Antioxidant, Attenuates Hyperglycemia and Downstream Complications. Metabolites 2022, 12, 1274. [Google Scholar] [CrossRef]
- Almatroodi, S.; Alnuqaydan, A.; Babiker, A.; Almogbel, M.; Khan, A.; Rahmani, A.H. 6-Gingerol, a Bioactive Compound of Ginger Attenuates Renal Damage in Streptozotocin-Induced Diabetic Rats by Regulating the Oxidative Stress and Inflammation. Pharmaceutics 2021, 13, 317. [Google Scholar] [CrossRef]
- Hughes, T.; Azim, S.; Ahmad, Z. Inhibition of Escherichia coli ATP synthase by dietary ginger phenolics. Int. J. Biol. Macromol. 2021, 182, 2130–2143. [Google Scholar] [CrossRef] [PubMed]
- Hayati, R.F.; Better, C.D.; Denis, D.; Komarudin, A.G.; Bowolaksono, A.; Yohan, B.; Sasmono, R.T. [6]-Gingerol Inhibits Chikungunya Virus Infection by Suppressing Viral Replication. BioMed Res. Int. 2021, 2021, 6623400. [Google Scholar] [CrossRef]
- Abolaji, A.O.; Ojo, M.; Afolabi, T.T.; Arowoogun, M.D.; Nwawolor, D.; Farombi, E.O. Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem. Biol. Interact. 2017, 270, 15–23. [Google Scholar] [CrossRef]
- Adetuyi, B.O.; Farombi, E.O. 6-Gingerol, an active constituent of ginger, attenuates lipopolysaccharide-induced oxidation, inflammation, cognitive deficits, neuroplasticity, and amyloidogenesis in rat. J. Food Biochem. 2021, 45, e13660. [Google Scholar] [CrossRef]
- Liao, Y.-R.; Leu, Y.-L.; Chan, Y.-Y.; Kuo, P.-C.; Wu, T.-S. Anti-Platelet Aggregation and Vasorelaxing Effects of the Constituents of the Rhizomes of Zingiber officinale. Molecules 2012, 17, 8928–8937. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, L.; Wang, S.; Chen, Z.; Han, T.; Ma, P.; Jiang, L.; Yuan, C.; Li, J.; Ke, D.; et al. 6-Gingerol Improves Ectopic Lipid Accumulation, Mitochondrial Dysfunction, and Insulin Resistance in Skeletal Muscle of Ageing Rats: Dual Stimulation of the AMPK/PGC-1α Signaling Pathway via Plasma Adiponectin and Muscular AdipoR1. Mol. Nutr. Food Res. 2019, 63, e1800649. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, Y.; Wang, T.; Wang, X.; Xu, Y.; Wang, Y.; Qiu, J. Ginger and 6-gingerol prevent lipopolysaccharide-induced intestinal barrier damage and liver injury in mice. J. Sci. Food Agric. 2021, 102, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Poltronieri, J.; Becceneri, A.B.; Fuzer, A.M.; Filho, J.C.C.; Martin, A.C.B.M.; Vieira, P.C.; Pouliot, N.; Cominetti, M.R. [6]-gingerol as a Cancer Chemopreventive Agent: A Review of Its Activity on Different Steps of the Metastatic Process. Mini Rev. Med. Chem. 2014, 14, 313–321. [Google Scholar] [CrossRef]
- Ahmed, S.H.H.; Gonda, T.; Hunyadi, A. Medicinal chemistry inspired by ginger: Exploring the chemical space around 6-gingerol. RSC Adv. 2021, 11, 26687–26699. [Google Scholar] [CrossRef]
- WHO. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 9 March 2023).
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2017, 38, 785–791. [Google Scholar] [CrossRef]
- Passacquale, G.; Sharma, P.; Perera, D.; Ferro, A. Antiplatelet therapy in cardiovascular disease: Current status and future directions. Br. J. Clin. Pharmacol. 2022, 88, 2686–2699. [Google Scholar] [CrossRef]
- Ornelas, A.; Zacharias-Millward, N.; Menter, D.G.; Davis, J.S.; Lichtenberger, L.; Hawke, D.; Hawk, E.; Vilar, E.; Bhattacharya, P.; Millward, S. Beyond COX-1: The effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 2017, 36, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Khodadi, E. Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules. Cardiovasc. Toxicol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Farhady, S.; Kobarfard, F.; Saghaie, L.; Rostami, M. Synthesis and Antiplatelet Activity Evaluation of a Group of Novel Ethyl Acetoacetate Phenylhydrazone Derivatives. Iran. J. Pharm. Res. 2021, 20, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, E.J.; Wills, B.K. Antiplatelet Drug Toxicity. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cortellini, G.; Caruso, C.; Romano, A. Aspirin challenge and desensitization: How, when and why. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 247–254. [Google Scholar] [CrossRef]
- Shim, Y.K.; Kim, N. Nonsteroidal Anti-inflammatory Drug and Aspirin-Induced Peptic Ulcer Disease. Korean J. Gastroenterol. 2016, 67, 300–312. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.J. The Influence of Herbal Medicine on Platelet Function and Coagulation: A Narrative Review. Semin. Thromb. Hemost. 2015, 41, 300–314. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.J. The Influence of Diet and Nutrients on Platelet Function. Semin. Thromb. Hemost. 2014, 40, 214–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abebe, W. Herbal medication: Potential for adverse interactions with analgesic drugs. J. Clin. Pharm. Ther. 2002, 27, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Mousa, S.A. Antithrombotic Effects of Naturally Derived Products on Coagulation and Platelet Function. Breast Cancer 2010, 663, 229–240. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Q.; Wang, F.-Q.; Li, C.-H.; Hu, Y.-J.; Xia, Z.-N.; Yang, F.-Q. In vitro anti-platelet aggregation effects of fourteen fruits and vegetables. Pak. J. Pharm. Sci. 2019, 32, 185–195. [Google Scholar]
- Young, H.-Y.; Liao, J.-C.; Chang, Y.-S.; Luo, Y.-L.; Lu, M.-C.; Peng, W.-H. Synergistic Effect of Ginger and Nifedipine on Human Platelet Aggregation: A Study in Hypertensive Patients and Normal Volunteers. Am. J. Chin. Med. 2006, 34, 545–551. [Google Scholar] [CrossRef]
- Bordia, A.; Verma, S.K.; Srivastava, K.C. Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot. Essent. Fat. Acids 1997, 56, 379–384. [Google Scholar] [CrossRef]
- Jiang, X.; Williams, K.M.; Liauw, W.S.; Ammit, A.J.; Roufogalis, B.D.; Duke, C.C.; Day, R.O.; McLachlan, A.J. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br. J. Clin. Pharmacol. 2005, 59, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Lumb, A.B. Effect of Dried Ginger on Human Platelet Function. Thromb. Haemost. 1994, 71, 110–111. [Google Scholar] [CrossRef]
- Shih, H.-C.; Chern, C.-Y.; Kuo, P.-C.; Wu, Y.-C.; Chan, Y.-Y.; Liao, Y.-R.; Teng, C.-M.; Wu, T.-S. Synthesis of Analogues of Gingerol and Shogaol, the Active Pungent Principles from the Rhizomes of Zingiber officinale and Evaluation of Their Anti-Platelet Aggregation Effects. Int. J. Mol. Sci. 2014, 15, 3926–3951. [Google Scholar] [CrossRef] [Green Version]
- Koo, K.L.; Ammit, A.J.; Tran, V.H.; Duke, C.C.; Roufogalis, B. Gingerols and Related Analogues Inhibit Arachidonic Acid-Induced Human Platelet Serotonin Release and Aggregation. Thromb. Res. 2001, 103, 387–397. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, P.; Zheng, B.; Zhang, M.; Zhang, Y.; Xue, Y.; Liu, C.; Chu, X.; Wang, X.; Sun, S.; et al. 6-Gingerol exerts a protective effect against hypoxic injury through the p38/Nrf2/HO-1 and p38/NF-κB pathway in H9c2 cells. J. Nutr. Biochem. 2022, 104, 108975. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-C.; Chang, Y.-H. Comparison of Inhibitory Capacities of 6-, 8- and 10-Gingerols/Shogaols on the Canonical NLRP3 Inflammasome-Mediated IL-1β Secretion. Molecules 2018, 23, 466. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Wang, Z.; Cai, L.; Lin, L.; Liu, J.; Cheng, J. NLRP3 Inflammasome and Its Central Role in the Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4293206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toldo, S.; Mezzaroma, E.; Buckley, L.F.; Potere, N.; Di Nisio, M.; Biondi-Zoccai, G.; Van Tassell, B.W.; Abbate, A. Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharmacol. Ther. 2022, 236, 108053. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-K.; Tsai, Y.-H.; Korinek, M.; Hung, P.-H.; El-Shazly, M.; Cheng, Y.-B.; Wu, Y.-C.; Hsieh, T.-J.; Chang, F.-R. 6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2017, 18, 168. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Ge, C.; Duan, D.; Zhang, B.; Cui, X.; Peng, S.; Liu, Y.; Fang, J. Activation of the Phase II Enzymes for Neuroprotection by Ginger Active Constituent 6-Dehydrogingerdione in PC12 Cells. J. Agric. Food Chem. 2014, 62, 5507–5518. [Google Scholar] [CrossRef]
- Kumboonma, P.; Senawong, T.; Saenglee, S.; Yenjai, C.; Phaosiri, C. Identification of phenolic compounds from Zingiber offinale and their derivatives as histone deacetylase inhibitors and antioxidants. Med. Chem. Res. 2017, 26, 650–661. [Google Scholar] [CrossRef]
- Murphree, S.S.; Mason, J.D.; Bean, T.G.; Perry, M.C. Rapid Aqueous Borohydride Reduction of Carbonyls Under Sealed-Tube Microwave Conditions. Synth. Commun. 2012, 42, 1979–1986. [Google Scholar] [CrossRef]
- Balaji, N.V.; Ramani, M.V.; Viana, A.G.; Sanglard, L.P.; White, J.; Mulabagal, V.; Lee, C.; Gana, T.J.; Egiebor, N.O.; Subbaraju, G.V.; et al. Design, synthesis and in vitro cell-based evaluation of the anti-cancer activities of hispolon analogs. Bioorg. Med. Chem. 2015, 23, 2148–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szakonyi, Z.; Gonda, T.; Ötvös, S.B.; Fülöp, F. Stereoselective syntheses and transformations of chiral 1,3-aminoalcohols and 1,3-diols derived from nopinone. Tetrahedron Asymmetry 2014, 25, 1138–1145. [Google Scholar] [CrossRef]
- Lin, Y.T.; Li, Y.; Hsu, H.C.; Tsai, J.Y.; Lee, J.H.; Tai, C.J.; Wu, M.J.; Wu, C.C. Discovery of 7, 4′-dimethoxy-3-hydroxyflavone as a protease-activated receptor 4 antagonist with antithrombotic activity and less bleeding tendency in mice. Biochem. Pharmacol. 2022, 202, 115152. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Mielnik, M.B.; Rzeszutek, A.; Triumf, E.C.; Egelansdal, B. Antioxidant and other quality properties of reindeer muscle from two different Norwegian regions. Meat Sci. 2011, 89, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Fási, L.; Latif, A.D.; Zupkó, I.; Lévai, S.; Dékány, M.; Béni, Z.; Könczöl, Á.; Balogh, G.T.; Hunyadi, A. AAPH or Peroxynitrite-Induced Biorelevant Oxidation of Methyl Caffeate Yields a Potent Antitumor Metabolite. Biomolecules 2020, 10, 1537. [Google Scholar] [CrossRef]
- Percepta; v2021.2.1 Build 3525; ACD/Labs: Toronto, ON, Canada, 2021.
- Marvin Sketch, Tautomer Generator; 20.21.0; Chemaxon: Budapest, Hungary, 2020.
- Avdeef, A. Permeability Equations. In Absorption and Drug Development: Solubility, Permeability, and Charge State; Avdeef, A., Ed.; Wiley Interscience: Hoboken, NJ, USA, 2012; pp. 465–481. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tóth, L.; Muszbek, L.; Komáromi, I. Mechanism of the irreversible inhibition of human cyclooxygenase-1 by aspirin as predicted by QM/MM calculations. J. Mol. Graph. Model. 2013, 40, 99–109. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeerch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Rychnovsky, S.D.; Skalitzky, D.J. Stereochemistry of alternating polyol chains: 13C NMR analysis of 1,3-diol acetonides. Tetrahedron Lett. 1990, 31, 945–948. [Google Scholar] [CrossRef]
- Lv, L.; Chen, H.; Soroka, M.; Chen, X.; Leung, T.; Sang, S. 6-Gingerdiols as the Major Metabolites of 6-Gingerol in Cancer Cells and in Mice and Their Cytotoxic Effects on Human Cancer Cells. J. Agric. Food Chem. 2012, 60, 11372–11377. [Google Scholar] [CrossRef] [Green Version]
- Kikuzaki, H.; Tsai, S.-M.; Nakatani, N. Gingerdiol related compounds from the rhizomes of Zingiber officinale. Phytochemistry 1992, 31, 1783–1786. [Google Scholar] [CrossRef]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery. ACS Chem. Neurosci. 2016, 7, 767–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25, Erratum in Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Tinworth, C.P.; Young, R.J. Facts, Patterns, and Principles in Drug Discovery: Appraising the Rule of 5 with Measured Physicochemical Data. J. Med. Chem. 2020, 63, 10091–10108. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G.; Rocca, B.; Patrono, C. The key contribution of platelet and vascular arachidonic acid metabolism to the pathophysiology of atherothrombosis. Cardiovasc. Res. 2021, 117, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Crescente, M.; Menke, L.; Chan, M.V.; Armstrong, P.C.; Warner, T.D. Eicosanoids in platelets and the effect of their modulation by aspirin in the cardiovascular system (and beyond). Br. J. Pharmacol. 2019, 176, 988–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurtjahja-Tjendraputra, E.; Ammit, A.; Roufogalis, B.; Tran, V.H.; Duke, C.C. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb. Res. 2003, 111, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Guh, J.-H.; Ko, F.-N.; Jong, T.-T.; Teng, C.-M. Antiplatelet Effect of Gingerol Isolated from Zingiber officinale. J. Pharm. Pharmacol. 1995, 47, 329–332. [Google Scholar] [CrossRef]
- Leeson, P.D.; Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 2007, 6, 881–890. [Google Scholar] [CrossRef]
- Tarcsay, Á.; Keserű, G.M. Contributions of Molecular Properties to Drug Promiscuity. J. Med. Chem. 2013, 56, 1789–1795. [Google Scholar] [CrossRef]




| Predicted Values a | Experimental Data | |||||||
|---|---|---|---|---|---|---|---|---|
| Cmpds ID | Tautomer Distribution (%) b | pKa,base/pKa,acid c | logP/logDpH7.4 | TPSA | HBD/HBA | CNS MPO d [62] | Kinetic Solubility e (μM) | PAMPA-BBB e’ Pe (·10−7 cm/s)/MR (%) |
| 1 | -/10.0 | 2.9/2.9 | 66.8 | 2/4 | 5.06 | >500 | 35.2 ± 2.4/23.8 ± 1.5 | |
| 2 | -/10.0 | 4.2/4.2 | 46.5 | 1/3 | 4.18 | 110.2 ± 3.8 | 31.7 ± 3.6/20.9 ± 3.9 | |
| 3 | -/10.0 | 4.1/4.1 | 46.5 | 1/3 | 4.19 | 45.2 ± 2.7 | -/11.0 ± 0.7 | |
| 4 | -/10.1 | 4.8/4.8 | 62.1 | 2/4 | 3.60 | 75.1 ± 7.3 | -/4.0 ± 11.1 | |
| 5 | -/10.1 | 3.0/3.0 | 69.9 | 3/4 | 4.72 | >500 | 33.9 ± 2,5/9.1 ± 7.6 | |
| 6 | -/10.1 | 3.0/3.0 | 69.9 | 3/4 | 4.72 | 460.7 ± 10.3 | 27.4 ± 1.6/18.5 ± 2.7 | |
| 11A | 31 | -/8.3 | 3.5/3.5 | 66.8 | 2/4 | 4.81 | 54.0 ± 1.3 | -/- |
| 11B | 60 | -/8.3 | 3.2/3.2 | 66.8 | 2/4 | 4.53 | ||
| 11C | 9 | -/8.7 | 3.3/3.3 | 63.6 | 1/4 | 5.01 | ||
| 12A | 38 | -/8.3 | 3.9/3.8 | 57.5 | 2/3 | 4.62 | 15.6 ± 0.1 | -/- |
| 12B | 54 | -/8.4 | 3.4/3.4 | 57.5 | 2/3 | 4.15 | ||
| 12C | 8 | -/8.9 | 3.5/3.5 | 54.4 | 1/3 | 4.75 | ||
| 13A | 31 | -/8.7 | 3.4/3.4 | 66.8 | 2/4 | 4.58 | 314.6 ± 23.7 | -/- |
| 13B | 60 | -/8.7 | 3.5/3.4 | 66.8 | 2/4 | 4.56 | ||
| 13C | 9 | -/9.4 | 3.3/3.3 | 63.6 | 1/4 | 4.98 | ||
| 14 | 3.8/10.1 | 4.7/4.7 | 58.1 | 2/4 | 3.67 | 276.7 ± 5.5 | 4.6 | |
| 15 | 3.9/10.1 | 4.9/4.9 | 48.9 | 2/3 | 3.57 | 202.9 ± 5.5 | 5.7 | |
| 16 | 3.1/10.0 | 4.8/4.8 | 48.9 | 2/3 | 3.59 | 89.4 ± 3.1 | -/- | |
| 17 | -/9.9 | 4.4/4.4 | 55.5 | 1/4 | 4.04 | 10.9 ± 2.0 | -/- | |
| 18 | -/9.8 | 4.8/4.8 | 46.3 | 1/3 | 3.87 | LOD | -/- | |
| 22 | -/10.1 | 3.6/3.6 | 58.6 | 2/4 | 4.36 | 482.6 ± 18.7 | 32.2 ± 3.4/12.1 ± 5.2 | |
| Aspirin | -/3.5 | 1.4/-1.7 | 63.6 | 1/4 | 5.75 | - | - | |
| Compound | Antiplatelet IC50 (µM) | COX-1 IC50 (µM) | ||
|---|---|---|---|---|
| 1 | 45.9 ± 5.1 | 1.46 | 62.5 ± 23.8 | 1.30 |
| 2 | 2.8 ± 0.5 | 1.40 | 9.8 ± 0.6 | 0.81 |
| 3 | 2.1 ± 1.0 | 1.56 | 4.4 ± 0.2 | 1.26 |
| 4 | 5.2 ± 0.4 | 0.47 | 5.2 ± 0.3 | 0.48 |
| 5 | 51.7 ± 2.7 | 1.26 | 54.3 ± 6.5 | 1.27 |
| 6 | 45.1 ± 6.0 | 1.32 | 76.2 ± 0.3 | 1.12 |
| 11(A) b | 4.1 ± 1.0 | 1.89 b | 23.1 ± 9.3 | 1.14 |
| 12(A) b | 71.7 ± 28.3 | 0.24 b | >200 | - |
| 13(B) b | 3.6 ± 0.9 | 1.94 b | 11.8 ± 5.4 | 1.53 |
| 14 | 4.1 ± 1.2 | 0.72 | 3.6 ± 0.2 | 0.74 |
| 15 | >100 | - | >200 | - |
| 16 | 3.5 ± 0.9 | 0.63 | 17.5 ± 0.1 | −0.04 |
| 17 | 3.1 ± 0.9 | 1.08 | 5.85 ± 0.04 | 0.83 |
| 18 | 32.0 ± 10.1 | −0.27 | >200 | - |
| 22 | 35.9 ± 23.7 | 0.80 | >100 | - |
| Aspirin | 106.0 ± 20.2 | 2.58 | - | - |
| DDPH | ORAC TE | ONOO– Scavenging (%) | XO Inhibition (%) | ||
|---|---|---|---|---|---|
| IC50 (µM) | LLE a | ||||
| 1 | 8.92 ± 0.46 | 2.15 | 1.30 ± 0.04 | <5.0 | <5.0 |
| 2 | 11.41 ± 0.49 | 0.75 | 1.10 ± 0.03 | <5.0 | <5.0 |
| 3 | 9.43 ± 0.16 | 0.93 | 1.36 ± 0.02 | <5.0 | <5.0 |
| 4 | 8.56 ± 0.07 | 0.27 | 0.47 ± 0.09 | <5.0 | 10.56 ± 2.30 |
| 5 | 6.51 ± 0.28 | 2.19 | 2.30 ± 0.05 | <5.0 | <5.0 |
| 6 | 13.82 ± 0.03 | 1.86 | 1.12 ± 0.03 | <5.0 | <5.0 |
| 11 | 9.04 ± 0.19 | 1.54 | 1.98 ± 0.12 | <5.0 | <5.0 |
| 12 | >200 | - | 2.89 ± 0.49 | <5.0 | <5.0 |
| 13 | 10.86 ± 0.69 | 1.46 | 2.60 ± 0.06 | <5.0 | <5.0 |
| 14 | 16.16 ± 0.46 | 0.09 | 0.44 ± 0.07 | <5.0 | 10.13 ± 1.65 |
| 15 | >200 | - | 1.00 ± 0.02 | <5.0 | 12.30 ± 0.90 |
| 16 | 18.98 ± 1.63 | −0.08 | 2.88 ± 0.16 | <5.0 | 16.01 ± 4.05 |
| 17 | 8.13 ± 0.21 | 0.69 | 0.77 ± 0.20 | 38.40 ± 3.05 | <5.0 |
| 18 | >100 | - | 1.76 ± 0.06 | 5.64 ± 1.31 | <5.0 |
| 22 | 14.07 ± 0.21 | 1.25 | 1.62 ± 0.004 | <5.0 | <5.0 |
| allopurinol | - | - | - | - | 98.80 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.H.H.; Gonda, T.; Agbadua, O.G.; Girst, G.; Berkecz, R.; Kúsz, N.; Tsai, M.-C.; Wu, C.-C.; Balogh, G.T.; Hunyadi, A. Preparation and Evaluation of 6-Gingerol Derivatives as Novel Antioxidants and Antiplatelet Agents. Antioxidants 2023, 12, 744. https://doi.org/10.3390/antiox12030744
Ahmed SHH, Gonda T, Agbadua OG, Girst G, Berkecz R, Kúsz N, Tsai M-C, Wu C-C, Balogh GT, Hunyadi A. Preparation and Evaluation of 6-Gingerol Derivatives as Novel Antioxidants and Antiplatelet Agents. Antioxidants. 2023; 12(3):744. https://doi.org/10.3390/antiox12030744
Chicago/Turabian StyleAhmed, Sara H. H., Tímea Gonda, Orinamhe G. Agbadua, Gábor Girst, Róbert Berkecz, Norbert Kúsz, Meng-Chun Tsai, Chin-Chung Wu, György T. Balogh, and Attila Hunyadi. 2023. "Preparation and Evaluation of 6-Gingerol Derivatives as Novel Antioxidants and Antiplatelet Agents" Antioxidants 12, no. 3: 744. https://doi.org/10.3390/antiox12030744
APA StyleAhmed, S. H. H., Gonda, T., Agbadua, O. G., Girst, G., Berkecz, R., Kúsz, N., Tsai, M. -C., Wu, C. -C., Balogh, G. T., & Hunyadi, A. (2023). Preparation and Evaluation of 6-Gingerol Derivatives as Novel Antioxidants and Antiplatelet Agents. Antioxidants, 12(3), 744. https://doi.org/10.3390/antiox12030744