Biodegradable Active Packaging Enriched with Essential Oils for Enhancing the Shelf Life of Strawberries
Abstract
:1. Introduction
2. Materials and Methods
2.1. PLA/PHB/ATBC Blend Preparation
2.2. Preparation of Polymer Solutions for Electrospinning
2.3. Electrospinning
2.4. Scanning Electron Microscopy (SEM)
2.5. Attenuated Total Reflectance—Fourier Tranform Infrared Spectrometry Analysis (ATR—FTIR)
2.6. Thermogravimetric Analysis (TG)
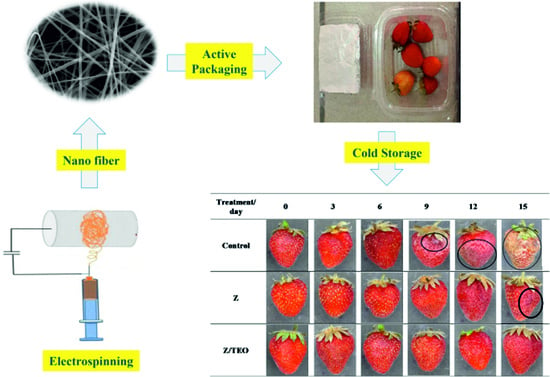
2.7. Application to Active Packaging of Strawberry
2.8. Quality Parameters
2.9. Total Phenolic Content and Antioxidant Activity
2.10. Total Anthocyanin Content
2.11. Microbiological Analysis
2.12. Sensory Evaluation
2.13. Statistical Analysis
3. Results and Discussion
3.1. SEM
3.2. FTIR
3.3. TGA
3.4. Fruit Quality Parameters
3.4.1. Color
3.4.2. Firmness
3.4.3. Soluble Solids Content
3.4.4. Weight Loss
3.5. Total Phenolic Content
3.6. Antioxidant Activity
3.7. Total Anthocyanin Content
3.8. Aerobic Mesophilic Bacteria, Psychrotrophic Bacteria, and Yeasts and Molds
3.9. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riesenegger, L.; Hübner, A. Reducing Food Waste at Retail Stores—An Explorative Study. Sustainability 2022, 14, 2494. [Google Scholar] [CrossRef]
- Ansarifar, E.; Moradinezhad, F. Encapsulation of Thyme Essential Oil Using Electrospun Zein Fiber for Strawberry Preservation. Chem. Biol. Technol. Agric. 2022, 9, 1–11. [Google Scholar] [CrossRef]
- Lombardi, A.; Fochetti, A.; Vignolini, P.; Campo, M.; Durazzo, A.; Lucarini, M.; Puglia, D.; Luzi, F.; Papalini, M.; Renzi, M. Natural Active Ingredients for Poly (Lactic Acid)—Based Materials: State of the Art and Perspectives. Antioxidants 2022, 11, 2074. [Google Scholar] [CrossRef] [PubMed]
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food—Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Lee, S.J.; Choi, D.S.; Hur, S.J. Current Topics in Active and Intelligent Food Packaging for Preservation of Fresh Foods. J. Sci. Food Agric. 2015, 95, 2799–2810. [Google Scholar] [CrossRef]
- Mosnáčková, K.; Opálková Šišková, A.; Kleinová, A.; Danko, M.; Mosnáček, J. Properties and Degradation of Novel Fully Biodegradable PLA/PHB Blends Filled with Keratin. Int. J. Mol. Sci. 2020, 21, 9678. [Google Scholar] [CrossRef]
- Mosnáčková, K.; Šlosár, M.; Kollár, J.; Janigová, I.; Šišková, A.; Chmela, Š.; Sikorska, W.; Perďochová, D.; Gálisová, I.; Alexy, P. Ageing of Plasticized Poly (Lactic Acid)/Poly (3-Hydroxybutyrate)/Carbon Black Mulching Films during One Season of Sweet Pepper Production. Eur. Polym. J. 2019, 114, 81–89. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (Lactic Acid) Blends: Processing, Properties and Applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of Active Packaging Film Made from Poly (Lactic Acid) Incorporated Essential Oil. Prog. Org. Coatings 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Min, T.; Sun, X.; Yuan, Z.; Zhou, L.; Jiao, X.; Zha, J.; Zhu, Z.; Wen, Y. Novel Antimicrobial Packaging Film Based on Porous Poly (Lactic Acid) Nanofiber and Polymeric Coating for Humidity-Controlled Release of Thyme Essential Oil. Lwt 2021, 135, 110034. [Google Scholar] [CrossRef]
- Napoli, E.M.; Ruberto, G. Sicilian Aromatic Plants: From Traditional Heritage to a New Agro-Industrial Exploitation. In Spices Types Uses Health Benefits; Kralis, J.F., Ed.; Nova Publishers: Hauppauge, NY, USA, 2012; pp. 1–56. [Google Scholar]
- Kapustová, M.; Puškárová, A.; Bučková, M.; Granata, G.; Napoli, E.; Annušová, A.; Mesárošová, M.; Kozics, K.; Pangallo, D.; Geraci, C. Biofilm Inhibition by Biocompatible Poly (ε-Caprolactone) Nanocapsules Loaded with Essential Oils and Their Cyto/Genotoxicity to Human Keratinocyte Cell Line. Int. J. Pharm. 2021, 606, 120846. [Google Scholar] [CrossRef]
- Kapustová, M.; Granata, G.; Napoli, E.; Puškárová, A.; Bučková, M.; Pangallo, D.; Geraci, C. Nanoencapsulated Essential Oils with Enhanced Antifungal Activity for Potential Application on Agri-Food, Material and Environmental Fields. Antibiotics 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The Antibacterial and Antifungal Activity of Six Essential Oils and Their Cyto/Genotoxicity to Human HEL 12469 Cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef] [Green Version]
- Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bučková, M.; Puškárová, A.; Kalászová, V.; Kisová, Z.; Pangallo, D. Essential Oils against Multidrug Resistant Gram-Negative Bacteria. Biologia 2018, 73, 803–808. [Google Scholar] [CrossRef]
- Napoli, E.; Di Vito, M. Toward a New Future for Essential Oils. Antibiotics 2021, 10, 207. [Google Scholar] [CrossRef]
- Karim, M.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of Cinnamic Aldehyde Using Zein Nanofibers by Novel Needle-Less Electrospinning: Production, Characterization and Their Application to Reduce Nitrite in Sausages. J. Food Eng. 2021, 288, 110140. [Google Scholar] [CrossRef]
- Colussi, R.; da Silva, W.M.F.; Biduski, B.; El Halal, S.L.M.; da Rosa Zavareze, E.; Dias, A.R.G. Postharvest Quality and Antioxidant Activity Extension of Strawberry Fruit Using Allyl Isothiocyanate Encapsulated by Electrospun Zein Ultrafine Fibers. LWT 2021, 143, 111087. [Google Scholar] [CrossRef]
- Gago, C.; Antão, R.; Dores, C.; Guerreiro, A.; Miguel, M.G.; Faleiro, M.L.; Figueiredo, A.C.; Antunes, M.D. The Effect of Nanocoatings Enriched with Essential Oils on ‘Rocha’Pear Long Storage. Foods 2020, 9, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gomes, M.d.S.; Cardoso, M.d.G.; Guimarães, A.C.G.; Guerreiro, A.C.; Gago, C.M.L.; Vilas Boas, E.V.d.B.; Dias, C.M.B.; Manhita, A.C.C.; Faleiro, M.L.; Miguel, M.G.C. Effect of Edible Coatings with Essential Oils on the Quality of Red Raspberries over Shelf-life. J. Sci. Food Agric. 2017, 97, 929–938. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols, Flavonoids and Antioxidant Activity of Aqueous and Methanolic Extracts of Propolis (Apis Mellifera L.) from Algarve, South Portugal. Food Sci. Technol. 2014, 34, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Yao, J.; Huang, S.; Long, X.; Wang, J.; García-García, E. Antioxidant Activity of Polyphenol and Anthocyanin Extracts from Fruits of Kadsura coccinea (Lem.) AC Smith. Food Chem. 2009, 117, 276–281. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Martinez, E.R.; Sekhon, J.K.; Vo, H. The Effect of Different Pressurized Fluids on the Extraction of Anthocyanins and Total Phenolics from Cranberry Pomace. J. Supercrit. Fluids 2021, 175, 105279. [Google Scholar] [CrossRef]
- NP-4405; Food Microbiology—General Rules for Microorganism Counts. Colonies Count at 30 °C. Instituto Português da Qualidade: Lisbon, Portugal, 2002.
- ISO 21527-2; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds; Part 2: Colony Count Technique in Products with Water Activity Less Than or Equal to 0.95. International Standards Organization: Geneva, Switzerland, 2008.
- Antunes, M.D.; Gago, C.; Guerreiro, A.; Sousa, A.R.; Julião, M.; Miguel, M.G.; Faleiro, M.L.; Panagopoulos, T. Nutritional Characterization and Storage Ability of Salicornia Ramosissima and Sarcocornia Perennis for Fresh Vegetable Salads. Horticulturae 2021, 7, 6. [Google Scholar] [CrossRef]
- Dhanalakshmi, M.; Jog, J.P. Preparation and Characterization of Electrospun Fibers of Nylon 11. Express Polym. Lett. 2008, 2, 540–545. [Google Scholar] [CrossRef]
- Opálková Šišková, A.; Pleva, P.; Hrůza, J.; Frajová, J.; Sedlaříková, J.; Peer, P.; Kleinová, A.; Janalíková, M. Reuse of Textile Waste to Production of the Fibrous Antibacterial Membrane with Filtration Potential. Nanomaterials 2022, 12, 50. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Perdiguero, M.; Fiori, S.; Kenny, J.M.; Peponi, L. Biodegradable Electrospun PLA-PHB Fibers Plasticized with Oligomeric Lactic Acid. Polym. Degrad. Stab. 2020, 179, 109226. [Google Scholar] [CrossRef]
- Hu, J.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Emulsion Electrospinning of Polycaprolactone: Influence of Surfactant Type towards the Scaffold Properties. J. Biomater. Sci. Polym. Ed. 2015, 26, 57–75. [Google Scholar] [CrossRef]
- Koombhongse, S.; Liu, W.; Reneker, D.H. Flat Polymer Ribbons and Other Shapes by Electrospinning. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2598–2606. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Wang, J.; Xu, Y. Effect of PBAT on Property of PLA/PHB Film Used for Fruits and Vegetables. Proc. MATEC Web Conf. EDP Sci. 2017, 88, 2009. [Google Scholar] [CrossRef] [Green Version]
- Fraj, A.; Jaâfar, F.; Marti, M.; Coderch, L.; Ladhari, N.A. Comparative Study of Oregano (Origanum vulgare L.) Essential Oil-Based Polycaprolactone Nanocapsules/Microspheres: Preparation, Physicochemical Characterization, and Storage Stability. Ind. Crops Prod. 2019, 140, 111669. [Google Scholar] [CrossRef]
- Ogede, R.O.; Abdulrahman, N.A. Density Functional Theory Study, Extraction and Characterization of Lemon Grass Oil (Cymbopogon citratus) as Antimalaria Repellant. World J. Adv. Res. Rev. 2022, 14, 284–297. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under Composting Conditions of Plasticized PLA–PHB Blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Sattary, M.; Amini, J.; Hallaj, R. Antifungal Activity of the Lemongrass and Clove Oil Encapsulated in Mesoporous Silica Nanoparticles against Wheat’s Take-All Disease. Pestic. Biochem. Physiol. 2020, 170, 104696. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, W.; Shen, C.; Dong, Q.; Wang, Y.; Zuo, S. Active Packaging Film Containing Oregano Essential Oil Microcapsules and Their Application for Strawberry Preservation. J. Food Process. Preserv. 2020, 44, e14799. [Google Scholar] [CrossRef]
- Ranjan, S.; Chandrasekaran, R.; Paliyath, G.; Lim, L.-T.; Subramanian, J. Effect of Hexanal Loaded Electrospun Fiber in Fruit Packaging to Enhance the Post Harvest Quality of Peach. Food Packag. Shelf Life 2020, 23, 100447. [Google Scholar] [CrossRef]
- Aharoni, Y.; Barkai-Golan, R. Pre-Harvest Fungicide Sprays and Polyvinyl Wraps to Control Botrytis Rot and Prolong the PostHarvest Storage Life of Strawberries. J. Hortic. Sci. 1987, 62, 177–181. [Google Scholar] [CrossRef]
- Amal, S.H.A.; El-Mogy, M.M.; Aboul-Anean, H.E.; Alsanius, B.W. Improving Strawberry Fruit Storability by Edible Coating as a Carrier of Thymol or Calcium Chloride. J. Hortic. Sci. Ornam. Plants 2010, 2, 88–97. [Google Scholar]
- Dhital, R.; Mora, N.B.; Watson, D.G.; Kohli, P.; Choudhary, R. Efficacy of Limonene Nano Coatings on Post-Harvest Shelf Life of Strawberries. Lwt 2018, 97, 124–134. [Google Scholar] [CrossRef]
- Chanjirakul, K.; Wang, S.Y.; Wang, C.Y.; Siriphanich, J. Effect of Natural Volatile Compounds on Antioxidant Capacity and Antioxidant Enzymes in Raspberries. Postharvest Biol. Technol. 2006, 40, 106–115. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.M.B.; Sargent, S.A. Physicochemical Changes during Strawberry Development in the Field Compared with Those That Occur in Harvested Fruit during Storage. J. Sci. Food Agric. 2006, 86, 180–190. [Google Scholar] [CrossRef]
- Gülçin, I.; Bursal, E.; Şehitoğlu, M.H.; Bilsel, M.; Gören, A.C. Polyphenol Contents and Antioxidant Activity of Lyophilized Aqueous Extract of Propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.-T.; Yin, J.-J. Effect of Allyl Isothiocyanate on Antioxidants and Fruit Decay of Blueberries. Food Chem. 2010, 120, 199–204. [Google Scholar] [CrossRef]
- Cordenunsi, B.R.; Oliveira do Nascimento, J.R.; Genovese, M.I.; Lajolo, F.M. Influence of Cultivar on Quality Parameters and Chemical Composition of Strawberry Fruits Grown in Brazil. J. Agric. Food Chem. 2002, 50, 2581–2586. [Google Scholar] [CrossRef]
- Bierhals, V.S.; Chiumarelli, M.; Hubinger, M.D. Effect of Cassava Starch Coating on Quality and Shelf Life of Fresh-cut Pineapple (Ananas comosus L. Merril Cv “Pérola”). J. Food Sci. 2011, 76, E62–E72. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.-T. Effect of Allyl Isothiocyanate on Antioxidant Enzyme Activities, Flavonoids and Post-Harvest Fruit Quality of Blueberries (Vaccinium corymbosum L., Cv. Duke). Food Chem. 2010, 122, 1153–1158. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of Antimicrobial Nanoemulsions as Edible Coatings: Impact on Safety and Quality Attributes of Fresh-Cut Fuji Apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.P.; Zhang, Y. Application of an Oregano Oil Nanoemulsion to the Control of Foodborne Bacteria on Fresh Lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.-H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A Novel Nano-Encapsulation Approach for Bioactive Compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Wu, H.; Zong, M.-H.; Jing, Y.-R.; Han, S.-Y. Encapsulation of Cinnamon Essential Oil in Electrospun Nanofibrous Film for Active Food Packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhu, Z.; Jiao, X.; Shang, Y.; Wen, Y. Encapsulation of Thymol in Biodegradable Nanofiber via Coaxial Eletrospinning and Applications in Fruit Preservation. J. Agric. Food Chem. 2019, 67, 1736–1741. [Google Scholar] [CrossRef] [PubMed]





| Quality Parameters | Treatments | Storage Duration (Days) | |||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | ||
| Lightness (L*) | Control | 42.06 ± 1.99 aA | 40.01 ± 1.65 bB | 40.58 ± 1.44 aB | 39.85 ± 1.21 aB |
| PLA/PHB/ATBC + 5% oregano EO | 42.06 ± 1.99 aA | 42.26 ± 0.57 aA | 39.86 ± 1.05 abB | 39.14 ± 0.87 aB | |
| PLA/PHB/ATBC + 5% lemongrass EO | 42.06 ± 1.99 aA | 39.12 ± 0.70 bB | 39.04 ± 0.96 bB | 37.54 ± 0.37 bB | |
| a* | Control | 32.49 ± 1.45 aB | 34.46 ± 0.72 bA | 34.25 ± 0.95 aA | 33.98 ± 1.08 aAB |
| PLA/PHB/ATBC + 5% oregano EO | 32.49 ± 1.45 aB | 35.51 ± 0.52 abA | 33.78 ± 0.34 aAB | 33.24 ± 0.11 aB | |
| PLA/PHB/ATBC + 5% lemongrass EO | 32.49 ± 1.45 aC | 35.85 ± 0.22 aA | 34.41 ± 0.57 aB | 33.64 ± 1.08 aBC | |
| b* | Control | 24.71 ± 2.14 aA | 25.16 ± 1.56 bA | 25.04 ± 1.86 aA | 24.58 ± 1.42 aA |
| PLA/PHB/ATBC + 5% oregano EO | 24.71 ± 2.14 aB | 28.06 ± 0.57 aA | 24.20 ± 0.89 aB | 24.40 ± 1.70 aB | |
| PLA/PHB/ATBC + 5% lemongrass EO | 24.71 ± 2.14 aA | 24.83 ± 1.08 bA | 23.04 ± 1.26 aA | 25.10 ± 5.50 aA | |
| Hue angle (h) | Control | 36.81 ± 1.46 aA | 36.02 ±1.54 bA | 35.85 ± 1.65 abA | 35.72 ± 0.70 aA |
| PLA/PHB/ATBC + 5% oregano EO | 36.81 ± 1.46 aA | 38.00 ± 0.75 aA | 35.27 ± 0.79 bA | 37.07 ± 3.62 aA | |
| PLA/PHB/ATBC + 5% lemongrass EO | 36.81 ± 1.46 aA | 34.51 ± 1.18 bAB | 33.64 ± 1.12 aC | 34.64 ± 2.48 aAB | |
| Chroma | Control | 41.01 ± 2.47 aA | 42.73 ± 1.31 bA | 42.37 ± 1.83 aA | 41.98 ± 1.71 aA |
| PLA/PHB/ATBC + 5% oregano EO | 41.01 ± 2.47 aB | 45.38 ± 0.51 aA | 41.64 ± 0.68 aB | 41.04 ± 0.74 aB | |
| PLA/PHB/ATBC + 5% lemongrass EO | 41.01 ± 2.47 aA | 43.68 ± 0.68 abA | 41.48 ± 1.09 aA | 42.77 ± 4.92 aA | |
| Firmness (N) | Control | 6.54 ± 0.35 aAB | 7.06 ± 0.23 abA | 5.89 ± 0.23 aB | 4.45 ± 0.62 aC |
| PLA/PHB/ATBC + 5% oregano EO | 6.54 ± 0.35 aB | 7.97 ± 1.71 aA | 6.20 ± 1.09 aB | 4.64 ± 0.81 aC | |
| PLA/PHB/ATBC + 5% lemongrass EO | 6.54 ± 0.35 aA | 6.42 ± 0.84 bA | 4.77 ± 0.50 bB | 3.32 ± 1.29 bC | |
| SSC (%) | Control | 7.15 ± 0.45 aA | 6.20 ± 0.55 aB | 6.23 ± 0.28 aB | 5.70 ± 0.37 aB |
| PLA/PHB/ATBC + 5% oregano EO | 7.15 ± 0.45 aA | 6.75 ± 0.17 aA | 6.28 ± 1.28 aA | 6.08 ± 0.30 aA | |
| PLA/PHB/ATBC + 5% lemongrass EO | 7.15 ± 0.45 aA | 6.35 ± 0.59 aB | 6.10 ± 0.39 aB | 6.13 ± 0.39 aB | |
| Weight loss (%) | Control | 2.19 ± 0.10 aC | 3.80 ± 0.22 aB | 5.42 ± 0.24 aA | |
| PLA/PHB/ATBC + 5% oregano EO | 1.50 ± 0.16 bC | 2.73 ± 0.34 bB | 4.27 ± 0.42 bA | ||
| PLA/PHB/ATBC + 5% lemongrass EO | 1.44 ± 0.06 bC | 2.54 ± 0.14 bB | 3.98 ± 0.24 bA | ||
| Total phenolic content (mg GAE.g−1) | Control | 86.28 ± 8.58 aC | 79.05 ± 11.84 bC | 233.51 ± 26.15 aB | 266.27 ± 17.47 aA |
| PLA/PHB/ATBC + 5% oregano EO | 86.28 ± 8.58 aB | 246.88 ± 91.56 aA | 220.32 ± 33.87 aA | 194.24 ± 102.46 aAB | |
| PLA/PHB/ATBC + 5% lemongrass EO | 86.28 ± 8.58 aB | 191.73 ± 75.47 aA | 201.28 ± 43.65 aA | 268.92 ± 69.43 aA | |
| Antioxidant activity (µM Trolox.g−1 FW) | Control | 172.86 ± 10.97 aA | 166.97 ± 14.76 aA | 167.34 ± 11.63 aA | 154.80 ± 10.52 aA |
| PLA/PHB/ATBC + 5% oregano EO | 172.86 ± 10.97 aA | 176.8 ± 7.86 aA | 175.18 ± 12.00 aA | 161.98 ± 8.45 aA | |
| PLA/PHB/ATBC + 5% lemongrass EO | 172.86 ± 10.97 aA | 164.26 ± 12.17 aA | 158.5 ± 16.98 aA | 150.54 ± 11.23 aA | |
| Total anthocyanin content (mg 100 g FW) | Control | 16.57 ± 3.04 aB | 9.53 ± 2.30 bC | 29.15 ± 1.47 aA | 29.49 ± 2.76 aA |
| PLA/PHB/ATBC + 5% oregano EO | 16.57 ± 3.04 aB | 17.31 ± 4.61 aB | 27.59 ± 3.92 aA | 21.55 ± 8.83 aA | |
| PLA/PHB/ATBC + 5% lemongrass EO | 16.57 ± 3.04 aB | 16.69 ± 5.40 aB | 30.39 ± 7.37 aA | 33.76 ± 8.91 aA | |
| Microorganisms (Log CFU/g) | Treatments | Storage Duration (days) | |||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | ||
| Yeast and molds | Control | 1.09 ± 0.11 aB | 2.36 ± 0.25 aA | 1.27 ± 0.85 aAB | 1.04 ± 1.21 aB |
| PLA/PHB/ATBC + 5% oregano EO | 1.09 ± 0.11 aA | 1.35 ± 0.91 abA | 1.27 ± 0.85 aA | 0.42 ± 0.85 aA | |
| PLA/PHB/ATBC + 5% lemongrass EO | 1.09 ± 0.11 aA | 0.92 ± 1.08 bA | 0.00 ± 0.00 bB | 0.00 ± 0.00 aB | |
| Aerobic mesophilic bacteria | Control | 0.84 ± 0.57 aA | 0.67 ± 0.47 aA | 0.89 ± 0.24 abA | 1.28 ± 0.21 abA |
| PLA/PHB/ATBC + 5% oregano EO | 0.84 ± 0.57 aA | 0.35 ± 0.40 aA | 0.75 ± 0.50 bA | 0.94 ± 0.63 bA | |
| PLA/PHB/ATBC + 5% lemongrass EO | 0.84 ± 0.57 aA | 1.32 ± 0.98 aA | 1.34 ± 0.23 aA | 1.65 ± 0.18 aA | |
| Taste Panels | Treatments | Evaluation |
|---|---|---|
| Fruit appearance | Control | 6.3 ± 0.73 |
| PLA/PHB/ATBC + 5% oregano EO | 6.3 ± 0.66 | |
| PLA/PHB/ATBC + 5% lemongrass EO | 6.0 ± 0.80 | |
| Aroma | Control | 5.15 ± 0.99 |
| PLA/PHB/ATBC + 5% oregano EO | 5.25 ± 1.16 | |
| PLA/PHB/ATBC + 5% lemongrass EO | 5.90 ± 0.91 | |
| Texture | Control | 5.70 ± 0.92 |
| PLA/PHB/ATBC + 5% oregano EO | 5.75 ± 1.07 | |
| PLA/PHB/ATBC + 5% lemongrass EO | 5.85 ± 1.14 | |
| Sweetness | Control | 4.7 ± 1.26 |
| PLA/PHB/ATBC + 5% oregano EO | 5.25 ± 1.48 | |
| PLA/PHB/ATBC + 5% lemongrass EO | 5.20 ± 1.40 | |
| Acidity | Control | 5.05 ± 1.00 |
| PLA/PHB/ATBC + 5% oregano EO | 5.45 ± 1.40 | |
| PLA/PHB/ATBC + 5% lemongrass EO | 5.35 ± 1.31 | |
| Flavor in general | Control | 5.15 ± 0.88 |
| PLA/PHB/ATBC + 5% oregano EO | 5.35 ± 1.46 | |
| PLA/PHB/ATBC + 5% lemongrass EO | 5.80 ± 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusková, M.; Opálková Šišková, A.; Mosnáčková, K.; Gago, C.; Guerreiro, A.; Bučková, M.; Puškárová, A.; Pangallo, D.; Antunes, M.D. Biodegradable Active Packaging Enriched with Essential Oils for Enhancing the Shelf Life of Strawberries. Antioxidants 2023, 12, 755. https://doi.org/10.3390/antiox12030755
Rusková M, Opálková Šišková A, Mosnáčková K, Gago C, Guerreiro A, Bučková M, Puškárová A, Pangallo D, Antunes MD. Biodegradable Active Packaging Enriched with Essential Oils for Enhancing the Shelf Life of Strawberries. Antioxidants. 2023; 12(3):755. https://doi.org/10.3390/antiox12030755
Chicago/Turabian StyleRusková, Magdaléna, Alena Opálková Šišková, Katarína Mosnáčková, Custódia Gago, Adriana Guerreiro, Mária Bučková, Andrea Puškárová, Domenico Pangallo, and Maria Dulce Antunes. 2023. "Biodegradable Active Packaging Enriched with Essential Oils for Enhancing the Shelf Life of Strawberries" Antioxidants 12, no. 3: 755. https://doi.org/10.3390/antiox12030755
APA StyleRusková, M., Opálková Šišková, A., Mosnáčková, K., Gago, C., Guerreiro, A., Bučková, M., Puškárová, A., Pangallo, D., & Antunes, M. D. (2023). Biodegradable Active Packaging Enriched with Essential Oils for Enhancing the Shelf Life of Strawberries. Antioxidants, 12(3), 755. https://doi.org/10.3390/antiox12030755