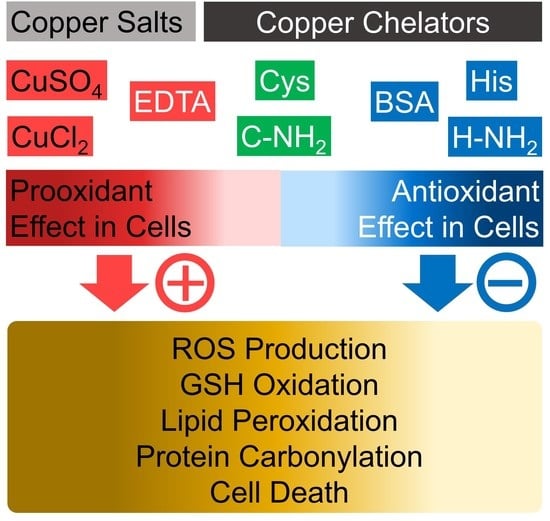
Differential Effects of Histidine and Histidinamide versus Cysteine and Cysteinamide on Copper Ion-Induced Oxidative Stress and Cytotoxicity in HaCaT Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Assay of Copper-Chelating Activity
2.3. Cell Culture
2.4. Assay of Cell Viability
2.5. Assay of ROS Production
2.6. Assay of GSH and Its Oxidized Form, Glutathione Disulfide (GSSG)
2.7. Preparation of Whole-Cell Lysates and Protein Assay
2.8. Assay of Lipid Peroxidation
2.9. Assay of Protein Carbonylation
2.10. Statistical Analysis
3. Results
3.1. Copper-Chelating Activities of Various Free and Amidated Amino Acids
3.2. CuSO4 Reduces Cell Viability While Increasing ROS Production of HaCaT Keratinocytes
3.3. Effects of Free and Amidated Amino Acids on HaCaT Cell Viability in the Absence and Presence of CuSO4
3.4. Dose-Dependent Effects of Histidine, Histidinamide, Cysteine, Cysteinamide, and EDTA on the CuSO4-Induced Death of HaCaT Cells
3.5. Effects of Histidine, Histidinamide, Cysteine, and Cysteinamide on the CuSO4-Induced ROS production in HaCaT Cells
3.6. Effects of Histidine, Histidinamide, Cysteine, and Cysteinamide on the GSH and GSSG Levels in HaCaT Cells Exposed to CuSO4
3.7. Effects of Histidine, Histidinamide, Cysteine, and Cysteinamide on the CuSO4-Induced Lipid Peroxidation and Protein Carbonylation in HaCaT Cells
3.8. Comparison of Copper-Chelating Activities of Cysteine, Cysteinamide, Histidine, Histidinamide, and BSA
3.9. Effects of Cysteine, Cysteinamide, Histidine, Histidinamide, and BSA on the Viabilities of HaCaT Cells Exposed to CuSO4 or CuCl2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chauhan, G.; Pant, K.K.; Nigam, K.D.P. Chelation technology: A promising green approach for resource management and waste minimization. Environ. Sci. Process. Impacts 2015, 17, 12–40. [Google Scholar] [CrossRef]
- Pinto, I.S.S.; Neto, I.F.F.; Soares, H.M.V.M. Biodegradable chelating agents for industrial, domestic, and agricultural applications-a review. Environ. Sci. Pollut. Res. 2014, 21, 11893–11906. [Google Scholar] [CrossRef]
- Franz, K.J. Clawing back: Broadening the notion of metal chelators in medicine. Curr. Opin. Chem. Biol. 2013, 17, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Hu, A.H.; Wilson, J.J. Advancing Chelation Strategies for Large Metal Ions for Nuclear Medicine Applications. Acc. Chem. Res. 2022, 55, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Tegoni, M.; Valensin, D.; Toso, L.; Remelli, M. Copper Chelators: Chemical Properties and Bio-medical Applications. Curr. Med. Chem. 2014, 21, 3785–3818. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Tishchenko, K.I.; Beloglazkina, E.K.; Mazhuga, A.G.; Zyk, N.V. Copper-containing enzymes: Site types and low-molecular-weight model compounds. Rev. J. Chem. 2016, 6, 49–82. [Google Scholar] [CrossRef]
- Wazir, S.M.; Ghobrial, I. Copper deficiency, a new triad: Anemia, leucopenia, and myeloneuropathy. J. Community Hosp. Intern. Med. Perspect. 2017, 7, 265–268. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L., Jr.; Adams, W.J.; Menzie, C.A. Critical Review of Exposure and Effects: Implications for Setting Regulatory Health Criteria for Ingested Copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef] [Green Version]
- Stern, B.R. Essentiality and Toxicity in Copper Health Risk Assessment: Overview, Update and Regulatory Considerations. J. Toxicol. Environ. Health-Part A-Curr. Issues 2010, 73, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [Green Version]
- Twomey, P.J.; Vijoen, A.; House, I.M.; Reynolds, T.M.; Wierzbicki, A.S. Relationship between serum copper, ceruloplasmin, and non-ceruloplasmin-bound copper in routine clinical practice. Clin. Chem. 2005, 51, 1558–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, A.N.; Xing, G.W.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Li, H.R.; Toh, P.Z.; Tan, J.Y.; Zin, M.T.; Lee, C.Y.; Li, B.; Leolukman, M.; Bao, H.Q.; Kang, L.F. Selected Biomarkers Revealed Potential Skin Toxicity Caused by Certain Copper Compounds. Sci. Rep. 2016, 6, 37664. [Google Scholar] [CrossRef] [Green Version]
- Shaligram, S.; Campbell, A. Toxicity of copper salts is dependent on solubility profile and cell type tested. Toxicol. Vitr. 2013, 27, 844–851. [Google Scholar] [CrossRef]
- Husain, N.; Mahmood, R. Copper(II) generates ROS and RNS, impairs antioxidant system and damages membrane and DNA in human blood cells. Environ. Sci. Pollut. Res. 2019, 26, 20654–20668. [Google Scholar] [CrossRef]
- Kang, Z.L.; Qiao, N.; Liu, G.Y.; Chen, H.M.; Tang, Z.X.; Li, Y. Copper-induced apoptosis and autophagy through oxidative stress-mediated mitochondrial dysfunction in male germ cells. Toxicol. Vitr. 2019, 61, 104639. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, I.P.; Kamal, A. Synthesis, Characterization of Some Complexes of Copper (II) with L-Asparginine, L-Histidine, L-Lysine. Am. J. Adv. Drug Deliv. 2015, 3, 95–103. [Google Scholar]
- Deschamps, P.; Kulkarni, P.P.; Gautam-Basak, M.; Sarkar, B. The saga of copper(II)-L-histidine. Coord. Chem. Rev. 2005, 249, 895–909. [Google Scholar] [CrossRef]
- Sheela, S.R.; Latha, M.; Liu, P.; Lem, K.; Kaler, S.G. Copper-replacement treatment for symptomatic Menkes disease: Ethical considerations. Clin. Genet. 2005, 68, 278–283. [Google Scholar] [CrossRef]
- Imer, F.; Aldemir, E.; Kilic, H.; Sonmezoglu, I.; Apak, R. The Protective Effect of Amino Acids on the Copper(II)-Catalyzed Autoxidation of Ascorbic Acid. J. Food Drug Anal. 2008, 16, 46–53. [Google Scholar] [CrossRef]
- Jiang, W.D.; Qu, B.; Feng, L.; Jiang, J.; Kuang, S.Y.; Wu, P.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q.; et al. Histidine Prevents Cu-Induced Oxidative Stress and the Associated Decreases in mRNA from Encoding Tight Junction Proteins in the Intestine of Grass Carp (Ctenopharyngodon idella). PLoS ONE 2016, 11, e0157001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timoshnikov, V.A.; Selyutina, O.Y.; Polyakov, N.E.; Didichenko, V.; Kontoghiorghes, G.J. Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. Int. J. Mol. Sci. 2022, 23, 1247. [Google Scholar] [CrossRef]
- Kim, J.H.; Seok, J.K.; Kim, Y.M.; Boo, Y.C. Identification of small peptides and glycinamide that inhibit melanin synthesis using a positional scanning synthetic peptide combinatorial library. Br. J. Derm. 2019, 181, 128–137. [Google Scholar] [CrossRef]
- Lee, H.K.; Ha, J.W.; Hwang, Y.J.; Boo, Y.C. Identification of L-Cysteinamide as a Potent Inhibitor of Tyrosinase-Mediated Dopachrome Formation and Eumelanin Synthesis. Antioxidants 2021, 10, 1202. [Google Scholar] [CrossRef]
- Lee, J.E.; Boo, Y.C. Combination of Glycinamide and Ascorbic Acid Synergistically Promotes Collagen Production and Wound Healing in Human Dermal Fibroblasts. Biomedicines 2022, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- Okajima, S.; Hamamoto, A.; Asano, M.; Isogawa, K.; Ito, H.; Kato, S.; Hirata, Y.; Furuta, K.; Takemori, H. Azepine derivative T4FAT, a new copper chelator, inhibits tyrosinase. Biochem. Biophys. Res. Commun. 2019, 509, 209–215. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blazquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [PubMed]
- Lai, W.W.; Hsiao, Y.P.; Chung, J.G.; Wei, Y.H.; Cheng, Y.W.; Yang, J.H. Synergistic phototoxic effects of glycolic acid in a human keratinocyte cell line (HaCaT). J. Derm. Sci. 2011, 64, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.W.; Boo, Y.C. Siegesbeckiae Herba Extract and Chlorogenic Acid Ameliorate the Death of HaCaT Keratinocytes Exposed to Airborne Particulate Matter by Mitigating Oxidative Stress. Antioxidants 2021, 10, 1762. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.A.; Ha, J.W.; Choi, J.Y.; Boo, Y.C. Antioxidant Effects of Korean Propolis in HaCaT Keratinocytes Exposed to Particulate Matter 10. Antioxidants 2022, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.A.; Barril, C.; Bedgood, D.R., Jr.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Huang, T.; Long, M.; Huo, B. Competitive Binding to Cuprous Ions of Protein and BCA in the Bicinchoninic Acid Protein Assay. Open Biomed. Eng. J. 2010, 4, 271–278. [Google Scholar] [CrossRef]
- Rakshit, A.; Khatua, K.; Shanbhag, V.; Comba, P.; Datta, A. Cu(2+) selective chelators relieve copper-induced oxidative stress in vivo. Chem. Sci. 2018, 9, 7916–7930. [Google Scholar] [CrossRef] [Green Version]
- Ohta, Y.; Shiraishi, N.; Nishikawa, T.; Nishikimi, M. Copper-catalyzed autoxidations of GSH and L-ascorbic acid: Mutual inhibition of the respective oxidations by their coexistence. Biochim. Biophys. Acta-Gen. Subj. 2000, 1474, 378–382. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.; Selyutina, O.Y.; Polyakov, N.E.; Kontoghiorghes, G.J. Effective inhibition of copper-catalyzed production of hydroxyl radicals by deferiprone. J. Biol. Inorg. Chem. 2019, 24, 331–341. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, M. Protein carbonylation: Molecular mechanisms, biological implications, and analytical approaches. Free Radic. Res. 2021, 55, 307–320. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Pei, R.N.; Zhang, Z.W.; Liao, J.Z.; Yu, W.L.; Qiao, N.; Han, Q.Y.; Li, Y.; Hu, L.M.; Guo, J.Y.; et al. Copper induces oxidative stress and apoptosis through mitochondria-mediated pathway in chicken hepatocytes. Toxicol. Vitr. 2019, 54, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.K.; Valko, M.; Cronin, M.T.D.; Jomová, K. Chelators in Iron and Copper Toxicity. Curr. Pharmacol. Rep. 2016, 2, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Harrington, J.M.; Boyd, W.A.; Smith, M.V.; Rice, J.R.; Freedman, J.H.; Crumbliss, A.L. Amelioration of Metal-Induced Toxicity in Caenorhabditis elegans: Utility of Chelating Agents in the Bioremediation of Metals. Toxicol. Sci. 2012, 129, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, M.H.; Ali, S.; Kamran, M.; Iqbal, N.; Azeem, M.; Javed, M.T.; Ali, Q.; Haider, M.Z.; Irshad, S.; Rizwan, M.; et al. Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities. Plants 2020, 9, 756. [Google Scholar] [CrossRef]
- Lin, C.C.; Su, T.H.; Wang, T.S. Protein carbonylation in THP-1 cells induced by cigarette smoke extract via a copper-catalyzed pathway. Chem. Res. Toxicol. 2009, 22, 1232–1238. [Google Scholar] [CrossRef]
- Yoshida, Y.; Furuta, S.; Niki, E. Effects of metal chelating agents on the oxidation of lipids induced by copper and iron. Biochim. Biophys. Acta 1993, 1210, 81–88. [Google Scholar] [CrossRef]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. GHK Peptide as a Natural Modulator of Multiple Cellular Pathways in Skin Regeneration. Biomed Res. Int. 2015, 2015, 648108. [Google Scholar] [CrossRef] [Green Version]
- Sheinenzon, A.; Shehadeh, M.; Michelis, R.; Shaoul, E.; Ronen, O. Serum albumin levels and inflammation. Int. J. Biol. Macromol. 2021, 184, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Hortin, G.L.; Landt, M.; Powderly, W.G. Changes in plasma amino acid concentrations in response to HIV-1 infection. Clin. Chem. 1994, 40, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Kirsipuu, T.; Zadoroznaja, A.; Smirnova, J.; Friedemann, M.; Plitz, T.; Tougu, V.; Palumaa, P. Copper(II)-binding equilibria in human blood. Sci. Rep. 2020, 10, 5686. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, J.W.; Choi, J.Y.; Boo, Y.C. Differential Effects of Histidine and Histidinamide versus Cysteine and Cysteinamide on Copper Ion-Induced Oxidative Stress and Cytotoxicity in HaCaT Keratinocytes. Antioxidants 2023, 12, 801. https://doi.org/10.3390/antiox12040801
Ha JW, Choi JY, Boo YC. Differential Effects of Histidine and Histidinamide versus Cysteine and Cysteinamide on Copper Ion-Induced Oxidative Stress and Cytotoxicity in HaCaT Keratinocytes. Antioxidants. 2023; 12(4):801. https://doi.org/10.3390/antiox12040801
Chicago/Turabian StyleHa, Jae Won, Joon Yong Choi, and Yong Chool Boo. 2023. "Differential Effects of Histidine and Histidinamide versus Cysteine and Cysteinamide on Copper Ion-Induced Oxidative Stress and Cytotoxicity in HaCaT Keratinocytes" Antioxidants 12, no. 4: 801. https://doi.org/10.3390/antiox12040801
APA StyleHa, J. W., Choi, J. Y., & Boo, Y. C. (2023). Differential Effects of Histidine and Histidinamide versus Cysteine and Cysteinamide on Copper Ion-Induced Oxidative Stress and Cytotoxicity in HaCaT Keratinocytes. Antioxidants, 12(4), 801. https://doi.org/10.3390/antiox12040801