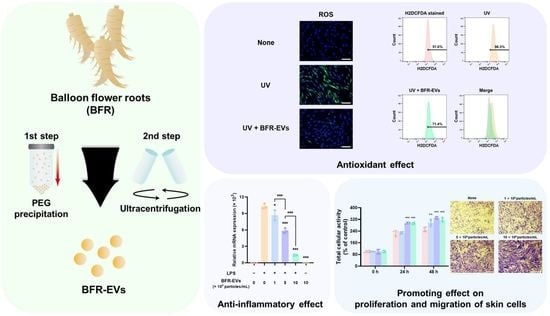
Balloon Flower Root-Derived Extracellular Vesicles: In Vitro Assessment of Anti-Inflammatory, Proliferative, and Antioxidant Effects for Chronic Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Isolation of BFR-EVs from BFR
2.3. Characterization of BFR-EVs
2.4. Cell Culture
2.5. Cytotoxicity Assessment of BFR-EVs
2.6. Cellular Uptake of BFR-EVs
2.7. Anti-Inflammation Activity Assay
2.8. Quantitative RT-PCR Analysis
2.9. Cell Proliferation Assay
2.10. Cell Migration Assay
2.11. Antioxidant Activity Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Characterization of BFR-EVs
3.2. Cytotoxic Effect and Cellular Uptake of BFR-EVs
3.3. Suppression Effect of BFR-EVs on Expression of Pro-Inflammatory Cytokines
3.4. Promotion Effects of BFR-EVs on Proliferation and Migration of HDFs
3.5. Antioxidant Effect of BFR-EVs on Oxidative Stress Induced by UV Irradiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joodaki, H.; Panzer, M.B. Skin mechanical properties and modeling: A review. Proc. Inst. Mech. Eng. H J. Eng. Med. 2018, 232, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Ghosh, K.; Tonnesen, M.G. Tissue engineering for cutaneous wounds. J. Investig. Dermatol. 2007, 127, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018, 3, 201–211. [Google Scholar] [CrossRef]
- Kurahashi, T.; Fujii, J. Roles of antioxidative enzymes in wound healing. J. Dev. Biol. 2015, 3, 57–70. [Google Scholar] [CrossRef]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 2018, 7, 98. [Google Scholar]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Moseley, R.; Leaver, M.; Walker, M.; Waddington, R.J.; Parsons, D.; Chen, W.Y.J.; Embery, G. Comparison of the antioxidant properties of HYAFF®-11p75, AQUACEL® and hyaluronan towards reactive oxygen species in vitro. Biomaterials 2002, 23, 2255–2264. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Xiong, Y.; Panayi, A.C.; Abududilibaier, A.; Hu, Y.; Yu, C.; Zhou, W.; Sun, Y.; Liu, M.; et al. Antioxidant Therapy and Antioxidant-Related Bionanomaterials in Diabetic Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 707479. [Google Scholar] [CrossRef]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The role of oxygen in wound healing: A review of the literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The antioxidant effect of small extracellular vesicles derived from aloe vera peels for wound healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv. Healthc. Mater. 2020, 9, 1901502. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Leu, J.-G.; Chen, S.-A.; Chen, H.-M.; Wu, W.-M.; Hung, C.-F.; Yao, Y.-D.; Tu, C.-S.; Liang, Y.-J. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 767–775. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, C.; Wang, Z.; Chen, W.; Liu, J.; Zhuo, R.; Yu, A.; Huang, S. A novel curcumin-loaded composite dressing facilitates wound healing due to its natural antioxidant effect. Drug Des. Devel. Ther. 2019, 13, 3269–3280. [Google Scholar] [CrossRef]
- Ma, T.; Zhai, X.; Huang, Y.; Zhang, M.; Zhao, X.; Du, Y.; Yan, C. A smart nanoplatform with photothermal antibacterial capability and antioxidant activity for chronic wound healing. Adv. Healthc. Mater. 2021, 10, 2100033. [Google Scholar] [CrossRef]
- Shiekh, P.A.; Singh, A.; Kumar, A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials 2020, 249, 120020. [Google Scholar] [CrossRef]
- Guan, Y.; Niu, H.; Liu, Z.; Dang, Y.; Shen, J.; Zayed, M.; Ma, L.; Guan, J. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci. Adv. 2021, 7, eabj0153. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, Y.; Ma, R.; Chen, J.; Qiu, J.; Du, S.; Li, C.; Wu, Z.; Yang, X.; Chen, Z. Thermosensitive hydrogel incorporating prussian blue nanoparticles promotes diabetic wound healing via ROS scavenging and mitochondrial function restoration. ACS Appl. Mater. Interfaces 2022, 14, 14059–14071. [Google Scholar] [CrossRef] [PubMed]
- Viaña-Mendieta, P.; Sánchez, M.L.; Benavides, J. Rational selection of bioactive principles for wound healing applications: Growth factors and antioxidants. Int. Wound J. 2022, 19, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, C.; Zheng, L.; Xia, L.; Li, Y.; Zhou, Y. The roles of extracellular vesicles in gastric cancer development, microenvironment, anti-cancer drug resistance, and therapy. Mol. Cancer 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.H. Isolation of Aloe saponaria-Derived Extracellular Vesicles and Investigation of Their Potential for Chronic Wound Healing. Pharmaceutics 2022, 14, 1905. [Google Scholar] [CrossRef]
- Kim, D.K.; Rhee, W.J. Antioxidative Effects of Carrot-Derived Nanovesicles in Cardiomyoblast and Neuroblastoma Cells. Pharmaceutics 2021, 13, 1203. [Google Scholar] [CrossRef]
- Savcı, Y.; Kırbaş, O.K.; Bozkurt, B.T.; Abdik, E.A.; Taşlı, P.N.; Şahin, F.; Abdik, H. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, S.E.; Seo, M.-J.; Kim, E.; Rhee, W.J. Suppression of inflammatory responses in macrophages by onion-derived extracellular vesicles. J. Ind. Eng. Chem. 2022, 115, 287–297. [Google Scholar] [CrossRef]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles as key mediators of plant–microbe interactions. Curr. Opin. Plant Biol. 2018, 44, 16–22. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Qasim, M.; Kim, J.-H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ma, J.; Zhou, Y.; Lu, R. Focusing on future applications and current challenges of plant derived extracellular vesicles. Pharmaceuticals 2022, 15, 708. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Mantel, P.-Y.; Halleck, A.E.; Trachtenberg, A.J.; Soria, C.E.; Oquin, S.; Bonebreak, C.M.; Saracoglu, E. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013, 394, 1253–1262. [Google Scholar] [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-derived extracellular vesicles: Recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 2022, 11, 12283. [Google Scholar] [CrossRef]
- Chen, C.J.; Liu, G.Y.; Shi, Y.T.; Zhu, C.S.; Pang, S.P.; Liu, X.S.; Ji, J. Biocompatible micelles based on comb-like PEG derivates: Formation, characterization, and photo-responsiveness. Macromol. Rapid Commun. 2011, 32, 1077–1081. [Google Scholar] [CrossRef]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and characterization of extracellular vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef]
- Ludwig, A.-K.; De Miroschedji, K.; Doeppner, T.R.; Börger, V.; Ruesing, J.; Rebmann, V.; Durst, S.; Jansen, S.; Bremer, M.; Behrmann, E. Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales. J. Extracell. Vesicles 2018, 7, 1528109. [Google Scholar] [CrossRef]
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G. ExtraPEG: A polyethylene glycol-based method for enrichment of extracellular vesicles. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Jang, K.-J.; Kim, H.K.; Han, M.H.; Oh, Y.N.; Yoon, H.-M.; Chung, Y.H.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Choi, Y.H. Anti-inflammatory effects of saponins derived from the roots of Platycodon grandiflorus in lipopolysaccharide-stimulated BV2 microglial cells. Int. J. Mol. Med. 2013, 31, 1357–1366. [Google Scholar] [CrossRef]
- Nyakudya, E.; Jeong, J.H.; Lee, N.K.; Jeong, Y.-S. Platycosides from the roots of Platycodon grandiflorum and their health benefits. Prev. Nutr. Food Sci. 2014, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, H.J. Phenolic Constituents from Platycodon grandiflorum root and their anti-inflammatory activity. Molecules 2021, 26, 4530. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Hwang, Y.P.; Kim, D.H.; Han, E.H.; Chung, Y.C.; Roh, S.H.; Jeong, H.G. Inhibitory effect of the saponins derived from roots of Platycodon grandiflorum on carrageenan-induced inflammation. Biosci. Biotechnol. Biochem. 2006, 70, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Yim, N.H.; Hwang, Y.H.; Liang, C.; Ma, J.Y. A platycoside-rich fraction from the root of Platycodon grandiflorum enhances cell death in A549 human lung carcinoma cells via mainly AMPK/mTOR/AKT signal-mediated autophagy induction. J. Ethnopharmacol. 2016, 194, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.S.; Kim, C.H.; Lee, S.Y.; Lee, K.S.; Choung, K.J.; Song, G.Y.; Kim, B.-H.; Ryu, S.Y.; Lee, H.S.; Kim, S.K. Evaluation of the total oxidant scavenging capacity of saponins isolated from Platycodon grandiflorum. Food Chem. 2012, 132, 333–337. [Google Scholar] [CrossRef]
- Lee, K.J.; Choi, C.Y.; Chung, Y.C.; Kim, Y.S.; Ryu, S.Y.; Roh, S.H.; Jeong, H.G. Protective effect of saponins derived from roots of Platycodon grandiflorum on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Toxicol. Lett. 2004, 147, 271–282. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, Y.S.; Yeo, S.J.; Roh, S.H.; Jeong, Y.C.; Kang, J.S.; Ryu, S.Y. Ameliorating effect of balloon flower saponin on the ethanol-induced memory impairment in mice. Phytother. Res. 2008, 22, 973–976. [Google Scholar] [CrossRef]
- Nam, Y.; Shin, S.J.; Park, Y.H.; Kim, M.-J.; Jeon, S.G.; Lee, H.; Choi, Y.; Kim, T.-J.; Shin, S.M.; Kim, J.-J. Platycodon grandiflorum Root Protects against Aβ-Induced Cognitive Dysfunction and Pathology in Female Models of Alzheimer’s Disease. Antioxidants 2021, 10, 207. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef]
- Van der Pol, E.; Coumans, F.; Grootemaat, A.; Gardiner, C.; Sargent, I.L.; Harrison, P.; Sturk, A.; Van Leeuwen, T.; Nieuwland, R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 2014, 12, 1182–1192. [Google Scholar] [CrossRef]
- Börger, V.; Staubach, S.; Dittrich, R.; Stambouli, O.; Giebel, B. Scaled Isolation of Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles. Curr. Protoc. Stem Cell Biol. 2020, 55, e128. [Google Scholar] [CrossRef]
- Das, S.; Baker, A.B. Biomaterials and nanotherapeutics for enhancing skin wound healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef]
- Chatterjee, S. Chapter Two—Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. [Google Scholar]
- Barbieri, S.S.; Eligini, S.; Brambilla, M.; Tremoli, E.; Colli, S. Reactive oxygen species mediate cyclooxygenase-2 induction during monocyte to macrophage differentiation: Critical role of NADPH oxidase. Cardiovasc. Res. 2003, 60, 187–197. [Google Scholar] [CrossRef]
- Onodera, Y.; Teramura, T.; Takehara, T.; Shigi, K.; Fukuda, K. Reactive oxygen species induce Cox-2 expression via TAK1 activation in synovial fibroblast cells. FEBS Open Bio 2015, 5, 492–501. [Google Scholar] [CrossRef]
- Yadav, E.; Singh, D.; Yadav, P.; Verma, A. Antioxidant and anti-inflammatory properties of Prosopis cineraria based phenolic rich ointment in wound healing. Biomed. Pharmacother. 2018, 108, 1572–1583. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Qureshi, K.A.; Ali, H.M.; Al-Omar, M.S.; Khan, O.; Mohammed, S.A. Bio-evaluation of the wound healing activity of Artemisia judaica L. as part of the plant’s use in traditional medicine; Phytochemical, antioxidant, anti-inflammatory, and antibiofilm properties of the plant’s essential oils. Antioxidants 2022, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.E.; Choi, S.S.; Park, T.H. Protective effects of silkworm hemolymph extract and its fractions on UV-induced photoaging. Biotechnol. Bioprocess Eng. 2017, 22, 37–44. [Google Scholar] [CrossRef]
- Svineng, G.; Ravuri, C.; Rikardsen, O.; Huseby, N.-E.; Winberg, J.-O. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect. Tissue Res. 2008, 49, 197–202. [Google Scholar] [CrossRef]
- Brenneisen, P.; Wenk, J.; Klotz, L.O.; Wlaschek, M.; Briviba, K.; Krieg, T.; Sies, H.; Scharffetter-Kochanek, K. Central role of Ferrous/Ferric iron in the ultraviolet B irradiation-mediated signaling pathway leading to increased interstitial collagenase (matrix-degrading metalloprotease (MMP)-1) and stromelysin-1 (MMP-3) mRNA levels in cultured human dermal fibroblasts. J. Biol. Chem. 1998, 273, 5279–5287. [Google Scholar]
- Alge-Priglinger, C.S.; Kreutzer, T.; Obholzer, K.; Wolf, A.; Mempel, M.; Kernt, M.; Kampik, A.; Priglinger, S.G. Oxidative stress-mediated induction of MMP-1 and MMP-3 in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5495–5503. [Google Scholar] [CrossRef] [PubMed]





| Gene (Accession #) | Primer | Sequence (5′-3′) |
|---|---|---|
| Mouse GAPDH (NC_000072.7) | Sense | GTC AGT GGT GGA CCT GAC CT |
| Antisense | TGC TGT AGC CAA ATT CGT TG | |
| IL-6 (NC_000007.14) | Sense | GCT ACC AAA CTG GAT ATA ATC GGA |
| Antisense | CCA GGT AGC TAT GGT ACT CCA GAA | |
| IL-1β (NC_000002.12) | Sense | AGT TGA CGG ACC CCA AAA G |
| Antisense | AGC TGG ATG CTC TCA TCA GG | |
| COX-2 (NC_012920.1) | Sense | GGG CTC AGC CAG GCA GCA AAT |
| Antisense | GCA CTG TGT TTG GGG TGG GCT | |
| Human GAPDH (NC_000012.12) | Sense | GTC AGT GGT GGA CCT GAC CT |
| Antisense | TGC TGT AGC CAA ATTCGT TG | |
| MMP-1 (NC_000011.10) | Sense | CAT CGT GTT GCA GCTCAT GA |
| Antisense | ATG GGCTGG ACA GGATTT TG | |
| MMP-3 (NC_000011.10) | Sense | TGC TGC TCA TGAAAT TGG CC |
| Antisense | TCA TCT TGA GACAGG CGG AA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Jang, H.; Park, J.H. Balloon Flower Root-Derived Extracellular Vesicles: In Vitro Assessment of Anti-Inflammatory, Proliferative, and Antioxidant Effects for Chronic Wound Healing. Antioxidants 2023, 12, 1146. https://doi.org/10.3390/antiox12061146
Kim M, Jang H, Park JH. Balloon Flower Root-Derived Extracellular Vesicles: In Vitro Assessment of Anti-Inflammatory, Proliferative, and Antioxidant Effects for Chronic Wound Healing. Antioxidants. 2023; 12(6):1146. https://doi.org/10.3390/antiox12061146
Chicago/Turabian StyleKim, Manho, Hyejun Jang, and Ju Hyun Park. 2023. "Balloon Flower Root-Derived Extracellular Vesicles: In Vitro Assessment of Anti-Inflammatory, Proliferative, and Antioxidant Effects for Chronic Wound Healing" Antioxidants 12, no. 6: 1146. https://doi.org/10.3390/antiox12061146
APA StyleKim, M., Jang, H., & Park, J. H. (2023). Balloon Flower Root-Derived Extracellular Vesicles: In Vitro Assessment of Anti-Inflammatory, Proliferative, and Antioxidant Effects for Chronic Wound Healing. Antioxidants, 12(6), 1146. https://doi.org/10.3390/antiox12061146