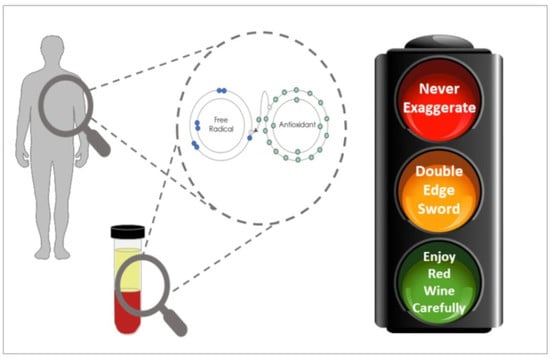
Oxidative Stress Is a Concept, Not an Indication for Selective Antioxidant Treatment
Abstract
:1. Introduction
2. Brief History of Redox Biology and Medicine
3. Hope
4. Identify-and-Treat Approach
5. Quantitation of Oxidative Stress
6. Assay Kits
7. Concluding Remarks
8. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| General terms | |
| GPx | Glutathione peroxidase |
| LMWA | Low-molecular-weight antioxidants |
| OS | Oxidative stress |
| PUFA | polyunsaturated fatty acid |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| Diseases | |
| AMD | Age-related macular degeneration |
| COPD | Chronic obstructive pulmonary disease |
| CVD | Cardiovascular disease |
| DM | Diabetes mellitus |
| PD | Parkinson’s disease |
| Indices | |
| AOPP | Advanced Oxidation Protein Products |
| CARR | Carrately |
| HO-1 | Heme oxygenase-1 |
| LMWA | Low-molecular-weight antioxidants |
| (ox)LDL | (oxidized) Low-density lipoprotein |
| MDA | Malondialdehyde |
| NT | Nitrotyrosine |
| PCOOH | Phosphatidylcholine Hydroperoxide |
| (d-)ROM | (determination of) Reactive oxygen metabolites |
| TAC | Total antioxidant capacity |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosin |
References
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, 31S–38S. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Pinchuk, I. Oxidative stress, the term and the concept. Biochem. Biophys. Res. Commun. 2015, 461, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Kyparos, A.; Spanou, C.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S. Redox biology of exercise: An integrative and comparative consideration of some overlooked issues. J. Exp. Biol. 2012, 215, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Dotan, Y.; Lichtenberg, D.; Pinchuk, I. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Prog. Lipid Res. 2004, 43, 200–227. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Pinchuk, I.; Weber, D. Oxidative stress, as assayed by a single test, cannot be used as a diagnostic tool. Biofactors 2018, 44, 222–223. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef]
- Miller, E.R., 3rd; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Ghezzi, P.; Jaquet, V.; Marcucci, F.; Schmidt, H.H. The oxidative stress theory of disease: Levels of evidence and epistemological aspects. Br. J. Pharmacol. 2017, 174, 1784–1796. [Google Scholar] [CrossRef]
- Pinchuk, I.; Kohen, R.; Stuetz, W.; Weber, D.; Franceschi, C.; Capri, M.; Hurme, M.; Grubeck-Loebenstein, B.; Schön, C.; Bernhardt, J.; et al. Do low molecular weight antioxidants contribute to the Protection against oxidative damage? The interrelation between oxidative stress and low molecular weight antioxidants based on data from the MARK-AGE study. Arch. Biochem. Biophys. 2021, 713, 109061. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.-S.; Najmeh Kaffash, F.; Mohammad Reza, M.-S.; Hedayatollah, S. Antioxidants as a Double-Edged Sword in the Treatment of Cancer. In Antioxidants; Emad, S., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 9. [Google Scholar] [CrossRef]
- Palmieri, B.; Sblendorio, V. Oxidative stress tests: Overview on reliability and use. Part I. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 309–342. [Google Scholar] [PubMed]
- Palmieri, B.; Sblendorio, V. Oxidative stress tests: Overview on reliability and use. Part II. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 383–399. [Google Scholar]
- Witztum, J.L. To E or not to E—How do we tell? Circulation 1998, 98, 2785–2787. [Google Scholar] [CrossRef]
- Bowry, V.W.; Stocker, R. Tocopherol-mediated peroxidation. The prooxidant effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J. Am. Chem. Soc. 1993, 115, 6029–6044. [Google Scholar] [CrossRef]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef]
- Di Minno, A.; Turnu, L.; Porro, B.; Squellerio, I.; Cavalca, V.; Tremoli, E.; Di Minno, M.N.D. 8-Hydroxy-2-Deoxyguanosine Levels and Cardiovascular Disease: A Systematic Review and Meta-Analysis of the Literature. Antioxid. Redox Signal. 2016, 24, 548–555. [Google Scholar] [CrossRef]
- Koenig, W.; Karakas, M.; Zierer, A.; Herder, C.; Baumert, J.; Meisinger, C.; Thorand, B. Oxidized LDL and the risk of coronary heart disease: Results from the MONICA/KORA Augsburg Study. Clin. Chem. 2011, 57, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Shi, D.; Lv, X.; Wang, B.; Chen, S.; Shao, Z. Prognostic significance of 8-hydroxy-2′-deoxyguanosine in solid tumors: A meta-analysis. BMC Cancer 2019, 19, 997. [Google Scholar] [CrossRef] [PubMed]
- Palla, V.V.; Karaolanis, G.; Katafigiotis, I.; Anastasiou, I.; Patapis, P.; Dimitroulis, D.; Perrea, D. gamma-H2AX: Can it be established as a classical cancer prognostic factor? Tumour Biol. 2017, 39, 1010428317695931. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Fois, A.G.; Sotgia, S.; A Mangoni, A.; Zinellu, E.; Pirina, P.; Carru, C.; Zinellu, A. Circulating malondialdehyde concentrations in patients with stable chronic obstructive pulmonary disease: A systematic review and meta-analysis. Biomark. Med. 2018, 12, 771–781. [Google Scholar] [CrossRef]
- Pinna, A.; Boscia, F.; Paliogiannis, P.; Carru, C.; Zinellu, A. Malondialdehyde Levels in Patients with Age-Related Macular Degeneration: A Systematic Review and Meta-analysis. Retina 2020, 40, 195–203. [Google Scholar] [CrossRef]
- Miyazawa, T.; Suzuki, T.; Fujimoto, K.; Kinoshita, M. Age-related change of phosphatidylcholine hydroperoxide and phosphatidylethanolamine hydroperoxide levels in normal human red blood cells. Mech. Ageing Dev. 1996, 86, 145–150. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, D.; Wang, M.; Zhao, F.; Han, X.; Qi, Y.; Liu, J. Association between Circulating Oxidized LDL and Atherosclerotic Cardiovascular Disease: A Meta-analysis of Observational Studies. Can. J. Cardiol. 2017, 33, 1624–1632. [Google Scholar] [CrossRef]
- Conti, G.; Caccamo, D.; Siligato, R.; Gembillo, G.; Satta, E.; Pazzano, D.; Carucci, N.; Carella, A.; Del Campo, G.; Salvo, A.; et al. Association of Higher Advanced Oxidation Protein Products (AOPPs) Levels in Patients with Diabetic and Hypertensive Nephropathy. Medicina 2019, 55, 675. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Pandey, K.B.; Abidi, A.B.; Rizvi, S.I. Markers of Oxidative Stress during Diabetes Mellitus. J. Biomark. 2013, 2013, 378790. [Google Scholar] [CrossRef]
- Moussa, S.A. Diabetes mellitus and oxidative stress. Rom. J. Biophys. 2008, 18, 225–236. [Google Scholar]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell. Adh Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef]
- Frankel, E.N. Antioxidants in Food and Biology Facts and Fiction; Press, O., Ed.; The Oily Press, Ltd.: Bridgwater, UK, 2007. [Google Scholar]
- Weber, D.; Stuetz, W.; Toussaint, O.; Debacq-Chainiaux, F.; Dollé, M.E.T.; Jansen, E.; Gonos, E.S.; Franceschi, C.; Sikora, E.; Hervonen, A.; et al. Associations between Specific Redox Biomarkers and Age in a Large European Cohort: The MARK-AGE Project. Oxidative Med. Cell. Longev. 2017, 2017, 1401452. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.; Weber, D.; Kochlik, B.; Stuetz, W.; Toussaint, O.; Debacq-Chainiaux, F.; Dollé, M.E.; Jansen, E.H.; Gonos, E.S.; Sikora, E.; et al. Gender- and age-dependencies of oxidative stress, as detected based on the steady state concentrations of different biomarkers in the MARK-AGE study. Redox Biol. 2019, 24, 101204. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Gebicki, J.; Puhl, H.; Jürgens, G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free. Radic. Biol. Med. 1992, 13, 341–390. [Google Scholar] [CrossRef] [PubMed]
- Atkin, M.A.; Gasper, A.; Ullegaddi, R.; Powers, H.J. Oxidative susceptibility of unfractionated serum or plasma: Response to antioxidants in vitro and to antioxidant supplementation. Clin. Chem. 2005, 51, 2138–2144. [Google Scholar] [CrossRef]
- Cadenas, E.; Sies, H. The lag phase. Free. Radic. Res. 1998, 28, 601–609. [Google Scholar] [CrossRef]
- Schnitzer, E.; Pinchuk, I.; Bor, A.; Fainaru, M.; Samuni, A.; Lichtenberg, D. Lipid oxidation in unfractionated serum and plasma. Chem. Phys. Lipids 1998, 92, 151–170. [Google Scholar] [CrossRef]
- Pinchuk, I.; Shoval, H.; Dotan, Y.; Lichtenberg, D. Evaluation of antioxidants: Scope, limitations and relevance of assays. Chem. Phys. Lipids 2012, 165, 638–647. [Google Scholar] [CrossRef]
- Pinchuk, I.; Lichtenberg, D. Prolongation of the lag time preceding peroxidation of serum lipids: A measure of antioxidant capacity. Methods Mol. Biol. 2015, 1208, 171–180. [Google Scholar] [CrossRef]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef]
- Trpkovic, A.; Resanovic, I.; Stanimirovic, J.; Radak, D.; Mousa, S.A.; Cenic-Milosevic, D.; Jevremovic, D.; Isenovic, E.R. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev.Clin. Lab. Sci. 2015, 52, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; De Felice, C.; Durand, T.; Oger, C.; Galano, J.M.; Leoncini, S.; Pecorelli, A.; Valacchi, G.; Ciccoli, L. Isoprostanes and 4-hydroxy-2-nonenal: Markers or mediators of disease? Focus on Rett syndrome as a model of autism spectrum disorder. Oxid. Med. Cell Longev. 2013, 2013, 343824. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kato, S.; Miyazawa, T. Determination of Phosphatidylcholine Hydroperoxide (PCOOH) as a Marker of Membrane Lipid Peroxidation. J Nutr Sci Vitaminol (Tokyo) 2015, 61, S78–S80. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.A.; Gut, A.L.; Azevedo, P.S.; Tanni, S.E.; Cunha, N.B.; Fernandes, A.A.H.; Polegato, B.F.; Zornoff, L.A.M.; de Paiva, S.A.R. Protein carbonyl concentration as a biomarker for development and mortality in sepsis-induced acute kidney injury. Biosci. Rep. 2018, 38, 1–8. [Google Scholar] [CrossRef]
- Cristani, M.; Speciale, A.; Saija, A.; Gangemi, S.; Minciullo, P.L.; Cimino, F. Circulating Advanced Oxidation Protein Products as Oxidative Stress Biomarkers and Progression Mediators in Pathological Conditions Related to Inflammation and Immune Dysregulation. Curr. Med. Chem. 2016, 23, 3862–3882. [Google Scholar] [CrossRef]
- Cipak Gasparovic, A.; Zarkovic, N.; Zarkovic, K.; Semen, K.; Kaminskyy, D.; Yelisyeyeva, O.; Bottari, S.P. Biomarkers of oxidative and nitro-oxidative stress: Conventional and novel approaches. Br. J. Pharmacol. 2017, 174, 1771–1783. [Google Scholar] [CrossRef]
- Chau, L.Y. Heme oxygenase-1: Emerging target of cancer therapy. J. Biomed. Sci. 2015, 22, 1–7. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, H.; Cho, E.J.; Youn, H.D. Ferritin binds and activates p53 under oxidative stress. Biochem. Biophys. Res. Commun. 2009, 389, 399–404. [Google Scholar] [CrossRef]
- Daiber, A.; Munzel, T. Organic Nitrate Therapy, Nitrate Tolerance, and Nitrate-Induced Endothelial Dysfunction: Emphasis on Redox Biology and Oxidative Stress. Antioxid. Redox. Signal. 2015, 23, 899–942. [Google Scholar] [CrossRef]
- Fayers, K.E.; Cummings, M.H.; Shaw, K.M.; Laight, D.W. Nitrate tolerance and the links with endothelial dysfunction and oxidative stress. Br. J. Clin. Pharmacol. 2003, 56, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, M.A.; Mendoza-Nunez, V.M. Oxidative Stress Indexes for Diagnosis of Health or Disease in Humans. Oxid. Med. Cell Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef] [PubMed]

| Pathology | Biomarker Found | Control (N) | Participants (N) | Reference |
|---|---|---|---|---|
| PD | 8-OHdG, MDA, nitrite, ferritin | 6037 | MA. 80 studies, N = 7212 | [21] |
| CVD | 8-OHdG OxLDL | 1106 1727 | MA. 14 studies, N = 810 prospective population-based case–cohort study, N = 333 | [22,23] |
| Cancer, solid tumors | 8-OHdG, γH2AX | MA. 21 studies, N = 2121 | [24,25] | |
| COPD | MDA | 530 | MA. 14 studies, N = 817 | [26] |
| AMD | MDA | 656 | MA. 12 studies, N = 634 | [27] |
| Aging | PCOOH | Young, 20 | Aged 20 | [28] |
| Atherosclerosis | oxLDL | MA. 12 studies, N = 8644 | [29] | |
| DM | AOPPs, MDA | 30 30 | DM patients 24 75 | [30,31,32] |
| Alzheimer’s disease | HO-1, 8-OHdG, HNE, isoprostanes, PCO, nitrotyrosine, AGEs | Review, 97 published articles | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lichtenberg, D.; Pinchuk, I.; Yonassi, E.; Weber, D.; Grune, T. Oxidative Stress Is a Concept, Not an Indication for Selective Antioxidant Treatment. Antioxidants 2023, 12, 1188. https://doi.org/10.3390/antiox12061188
Lichtenberg D, Pinchuk I, Yonassi E, Weber D, Grune T. Oxidative Stress Is a Concept, Not an Indication for Selective Antioxidant Treatment. Antioxidants. 2023; 12(6):1188. https://doi.org/10.3390/antiox12061188
Chicago/Turabian StyleLichtenberg, Dov, Ilya Pinchuk, Eleni Yonassi, Daniela Weber, and Tilman Grune. 2023. "Oxidative Stress Is a Concept, Not an Indication for Selective Antioxidant Treatment" Antioxidants 12, no. 6: 1188. https://doi.org/10.3390/antiox12061188
APA StyleLichtenberg, D., Pinchuk, I., Yonassi, E., Weber, D., & Grune, T. (2023). Oxidative Stress Is a Concept, Not an Indication for Selective Antioxidant Treatment. Antioxidants, 12(6), 1188. https://doi.org/10.3390/antiox12061188