Phaeanthus vietnamensis Ban Ameliorates Lower Airway Inflammation in Experimental Asthmatic Mouse Model via Nrf2/HO-1 and MAPK Signaling Pathway
Abstract
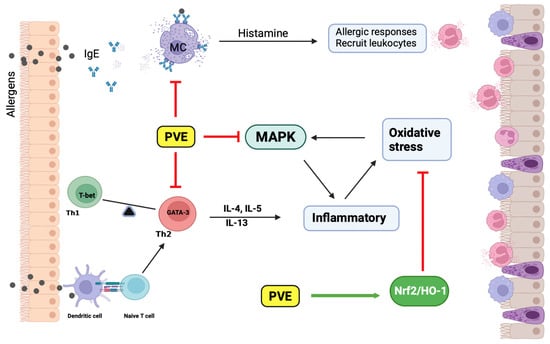
:1. Introduction
2. Materials and Methods
2.1. Phaeanthus vietnamensis Extract Preparation
2.2. Chemical Constituents PVE Analyzed through UPLC-Q-TOF-MS/MS
2.3. Animal Protocol
2.4. Cytotoxicity MTT Assay
2.5. Rat Peritoneal Mast Cells (RPMCs) Degranulation Assay
2.6. Bronchoalveolar Lavage Fluid Analysis
2.7. Histopathology and Immunohistochemistry
2.8. Measurement of Immunoglobulins and Cytokine Using ELISA
2.9. Western Blot
2.10. Statistical Analysis
3. Results
3.1. Analyzed Compounds from Phaeanthus vietnamensis
3.2. PVE Is Non-Toxic to the Rat Peritoneal Mast Cell (RPMC)
3.3. PVE Prevented Degranulation of RPMCs by Compound 48/80
3.4. PVE Reduced the Recruitment of Inflammatory Cells in BALF of the OVA-Induced Asthmatic Mouse Model
3.5. PVE Reduced Asthma Histopathological Features in Lung Tissue of the OVA-Induced Asthmatic Mouse Model
3.6. PVE Reduced The Levels of OVA-Specific Antibodies and Histamine in the Serum of the OVA-Induced Asthmatic Mouse Model
3.7. PVE Restored the Balance of Helper T Cell Responses in the BALF of the OVA-Induced Asthmatic Mouse Model
3.8. Effect of PVE on Oxidative Stress in the OVA-Induced Asthmatic Mouse Model
3.9. PVE Inactivates the MAPK Signaling Pathway in the OVA-Induced Asthmatic Mouse Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enilari, O.; Sinha, S. The Global Impact of Asthma in Adult Populations. Ann. Glob. Health 2019, 85, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shifren, A.; Witt, C.; Christie, C.; Castro, M. Mechanisms of remodeling in asthmatic airways. J. Allergy 2012, 2012, 316049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, J. The role of mast cells in the pathophysiology of asthma. N. Engl. J. Med. 2002, 346, 1742–1743. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.S.; Babu, K.S.; Holgate, S.T. Is asthma really due to a polarized T cell response toward a helper T cell type 2 phenotype? Am. J. Respir. Crit. Care Med. 2001, 164, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xin, J.; Coleman, J.; Huang, H. IFN-gamma suppresses STAT6 phosphorylation by inhibiting its recruitment to the IL-4 receptor. J. Immunol. 2005, 174, 1332–1337. [Google Scholar] [CrossRef] [Green Version]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, O. Oxidative stress in asthma: Part of the puzzle. Pediatr. Allergy Immunol. 2018, 29, 789–800. [Google Scholar] [CrossRef]
- Cherian, D.A.; Peter, T.; Narayanan, A.; Madhavan, S.S.; Achammada, S.; Vynat, G.P. Malondialdehyde as a Marker of Oxidative Stress in Periodontitis Patients. J. Pharm. Bioallied Sci. 2019, 11, S297–S300. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjornsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.O.; Song, K.H.; Lee, I.S.; Lee, S.J.; Kim, W.I.; Pak, S.W.; Shin, I.S.; Kim, T. Cimicifugae Rhizoma Extract Attenuates Oxidative Stress and Airway Inflammation via the Upregulation of Nrf2/HO-1/NQO1 and Downregulation of NF-kappaB Phosphorylation in Ovalbumin-Induced Asthma. Antioxidants 2021, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, T.; Guo, J.; Mitzner, W.A.; Roman, J.; Singh, A.; Fryer, A.D.; Yamamoto, M.; Kensler, T.W.; Tuder, R.M.; Georas, S.N.; et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005, 202, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Efficacy of inhaled corticosteroids in asthma. J. Allergy Clin. Immunol. 1998, 102, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Goyal, A.; Bansal, P.; Sonthalia, S. Corticosteroid Adverse Effects. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531462/ (accessed on 13 June 2023).
- Ryu, J.H.; Kim, Y.; Kim, T.; Kim, Y.M.; Jung, J.; Lee, S.-Y.; Lee, S.E.; Kim, N.G.; Shin, Y.-I. Light-emitting diode-based photobiomodulation reduces features of allergic asthma in mice. Allergy 2020, 75, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Chi, V.V. Dictionary of medicinal plants in Vietnam. Med. Publ. House Hanoi 2012, 1, 441–442. [Google Scholar]
- Tu, D.N.; Hau, V.T.B.; Diep, N.T.; Khanh, H.V.; Long, N.T.; Trang, H.T.H.; Hoang, N.H.; Kiem, P.V.; Nhiem, N.X. Alkaloids from Phaeanthus vietnamensis with inhibitory effect on nitric oxide production lipopolysaccharide-stimulated in RAW264.7 macrophages. J. Asian Nat. Prod. Res. 2021, 24, 898–903. [Google Scholar] [CrossRef]
- Nhiem, N.X.; Tuong, N.T.; Ky, P.T.; Subedi, L.; Park, S.J.; Ngoc, T.M.; Yen, P.H.; Tai, B.H.; Quang, T.H.; Kiem, P.V.; et al. Chemical Components from Phaeanthus vietnamensis and Their Inhibitory NO Production in BV2 Cells. Chem. Biodivers. 2017, 14, e1700013. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Correction to: Neophytadiene from Turbinaria ornata Suppresses LPS-Induced Inflammatory Response in RAW 264.7 Macrophages and Sprague Dawley Rats. Inflammation 2020, 43, 951–952. [Google Scholar] [CrossRef] [Green Version]
- do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.D.C.; Gois Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Liang, Q.; Yang, J.; He, J.; Chen, X.; Zhang, H.; Jia, M.; Liu, K.; Jia, C.; Pan, Y.; Wei, J. Stigmasterol alleviates cerebral ischemia/reperfusion injury by attenuating inflammation and improving antioxidant defenses in rats. Biosci. Rep. 2020, 40, BSR20192133. [Google Scholar] [CrossRef] [Green Version]
- Giorno, T.B.S.; Lima, F.A.; Brand, A.L.M.; Oliveira, C.M.; Rezende, C.M.; Fernandes, P.D. Characterization of (beta)N-Octadecanoyl-5-hydroxytryptamide Anti-Inflammatory Effect. Molecules 2021, 26, 3709. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [PubMed]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Piao, C.H.; Song, C.H.; Shin, H.S.; Shon, D.H.; Chai, O.H. Piper nigrum extract ameliorated allergic inflammation through inhibiting Th2/Th17 responses and mast cells activation. Cell Immunol. 2017, 322, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yan, G.H.; Chai, O.H.; Choi, Y.H.; Zhang, X.; Lim, J.M.; Kim, J.H.; Lee, M.S.; Han, E.H.; Kim, H.T.; et al. Inhibitory effects of Agaricus blazei on mast cell-mediated anaphylaxis-like reactions. Biol. Pharm. Bull. 2006, 29, 1366–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bui, T.T.; Piao, C.H.; Kim, S.M.; Song, C.H.; Shin, H.S.; Lee, C.H.; Chai, O.H. Citrus tachibana Leaves Ethanol Extract Alleviates Airway Inflammation by the Modulation of Th1/Th2 Imbalance via Inhibiting NF-kappaB Signaling and Histamine Secretion in a Mouse Model of Allergic Asthma. J. Med. Food 2017, 20, 676–684. [Google Scholar] [CrossRef]
- BArIS, S.A.; VurAL, C.; YAPrAK, B.; OnYILMAZ, T.; TunCEL, D.; Vatansever, S.; Isken, T.; BASYIgIT, I.; Boyaci, H.; Yildiz, F. The effects of sildenafil on smoke induced lung inflammation in rats. Malays. J. Pathol. 2016, 38, 39. [Google Scholar]
- Lee, S.Y.; Bae, C.S.; Seo, N.S.; Na, C.S.; Yoo, H.Y.; Oh, D.S.; Bae, M.S.; Kwon, M.S.; Cho, S.S.; Park, D.H. Camellia japonica oil suppressed asthma occurrence via GATA-3 & IL-4 pathway and its effective and major component is oleic acid. Phytomedicine 2019, 57, 84–94. [Google Scholar] [CrossRef]
- Medeiros-de-Moraes, I.M.; Goncalves-de-Albuquerque, C.F.; Kurz, A.R.M.; Oliveira, F.M.J.; de Abreu, V.H.P.; Torres, R.C.; Carvalho, V.F.; Estato, V.; Bozza, P.T.; Sperandio, M.; et al. Omega-9 Oleic Acid, the Main Compound of Olive Oil, Mitigates Inflammation during Experimental Sepsis. Oxid. Med. Cell Longev. 2018, 2018, 6053492. [Google Scholar] [CrossRef] [Green Version]
- Toda, T.; Yoshino, S. Enhancement of ovalbumin-specific Th1, Th2, and Th17 immune responses by amorphous silica nanoparticles. Int. J. Immunopathol. Pharmacol. 2016, 29, 408–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, A.; Khare, A.; Krishnamoorthy, N.; Qi, Z.; Ray, P. Regulatory T cells in many flavors control asthma. Mucosal. Immunol. 2010, 3, 216–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mountford, A.P.; Fisher, A.; Wilson, R.A. The profile of IgG1 and IgG2a antibody responses in mice exposed to Schistosoma mansoni. Parasite Immunol. 1994, 16, 521–527. [Google Scholar] [CrossRef]
- Tanabe, T.; Fujimoto, K.; Yasuo, M.; Tsushima, K.; Yoshida, K.; Ise, H.; Yamaya, M. Modulation of mucus production by interleukin-13 receptor alpha2 in the human airway epithelium. Clin. Exp. Allergy 2008, 38, 122–134. [Google Scholar] [CrossRef]
- Kudo, M.; Ishigatsubo, Y.; Aoki, I. Pathology of asthma. Front. Microbiol. 2013, 4, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S. Chapter two-oxidative stress, inflammation, and disease. Oxid. Stress Biomater. 2016, 35–58. [Google Scholar] [CrossRef]
- Cho, Y.S.; Moon, H.B. The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol. Res. 2010, 2, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Heck, D.E.; Shakarjian, M.; Kim, H.D.; Laskin, J.D.; Vetrano, A.M. Mechanisms of oxidant generation by catalase. Ann. N. Y. Acad. Sci. 2010, 1203, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.; Maier, O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxid. Med. Cell Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef] [Green Version]
- Wood, L.G.; Gibson, P.G.; Garg, M.L. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur. Respir. J. 2003, 21, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Biswas, C.; Shah, N.; Muthu, M.; La, P.; Fernando, A.P.; Sengupta, S.; Yang, G.; Dennery, P.A. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J. Biol. Chem. 2014, 289, 26882–26894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezatabar, S.; Karimian, A.; Rameshknia, V.; Parsian, H.; Majidinia, M.; Kopi, T.A.; Bishayee, A.; Sadeghinia, A.; Yousefi, M.; Monirialamdari, M.; et al. RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. J. Cell Physiol. 2019, 234, 14951–14965. [Google Scholar] [CrossRef]
- Kim, H.K. Role of ERK/MAPK signalling pathway in anti-inflammatory effects of Ecklonia cava in activated human mast cell line-1 cells. Asian Pac. J. Trop. Med. 2014, 7, 703–708. [Google Scholar] [CrossRef] [Green Version]
- Maneechotesuwan, K.; Xin, Y.; Ito, K.; Jazrawi, E.; Lee, K.Y.; Usmani, O.S.; Barnes, P.J.; Adcock, I.M. Regulation of Th2 cytokine genes by p38 MAPK-mediated phosphorylation of GATA-3. J. Immunol. 2007, 178, 2491–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, P.R.; McLaughlin, M.M.; Kumar, S.; Kassis, S.; Doyle, M.L.; McNulty, D.; Gallagher, T.F.; Fisher, S.; McDonnell, P.C.; Carr, S.A.; et al. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J. Biol. Chem. 1997, 272, 12116–12121. [Google Scholar] [CrossRef] [Green Version]
- Bennett, B.L. c-Jun N-terminal kinase-dependent mechanisms in respiratory disease. Eur. Respir. J. 2006, 28, 651–661. [Google Scholar] [CrossRef]
- Li, S.T.; Dai, Q.; Zhang, S.X.; Liu, Y.J.; Yu, Q.Q.; Tan, F.; Lu, S.H.; Wang, Q.; Chen, J.W.; Huang, H.Q.; et al. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-kappaB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol. Sin. 2018, 39, 1294–1304. [Google Scholar] [CrossRef]
- Duan, W.; Wong, W.S. Targeting mitogen-activated protein kinases for asthma. Curr. Drug Targets 2006, 7, 691–698. [Google Scholar] [CrossRef] [PubMed]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.V.; Vo, C.T.; Vo, V.M.; Nguyen, C.T.T.; Pham, T.M.; Piao, C.H.; Fan, Y.J.; Chai, O.H.; Bui, T.T. Phaeanthus vietnamensis Ban Ameliorates Lower Airway Inflammation in Experimental Asthmatic Mouse Model via Nrf2/HO-1 and MAPK Signaling Pathway. Antioxidants 2023, 12, 1301. https://doi.org/10.3390/antiox12061301
Nguyen TV, Vo CT, Vo VM, Nguyen CTT, Pham TM, Piao CH, Fan YJ, Chai OH, Bui TT. Phaeanthus vietnamensis Ban Ameliorates Lower Airway Inflammation in Experimental Asthmatic Mouse Model via Nrf2/HO-1 and MAPK Signaling Pathway. Antioxidants. 2023; 12(6):1301. https://doi.org/10.3390/antiox12061301
Chicago/Turabian StyleNguyen, Thi Van, Chau Tuan Vo, Van Minh Vo, Cong Thuy Tram Nguyen, Thi My Pham, Chun Hua Piao, Yan Jing Fan, Ok Hee Chai, and Thi Tho Bui. 2023. "Phaeanthus vietnamensis Ban Ameliorates Lower Airway Inflammation in Experimental Asthmatic Mouse Model via Nrf2/HO-1 and MAPK Signaling Pathway" Antioxidants 12, no. 6: 1301. https://doi.org/10.3390/antiox12061301
APA StyleNguyen, T. V., Vo, C. T., Vo, V. M., Nguyen, C. T. T., Pham, T. M., Piao, C. H., Fan, Y. J., Chai, O. H., & Bui, T. T. (2023). Phaeanthus vietnamensis Ban Ameliorates Lower Airway Inflammation in Experimental Asthmatic Mouse Model via Nrf2/HO-1 and MAPK Signaling Pathway. Antioxidants, 12(6), 1301. https://doi.org/10.3390/antiox12061301