Enhanced Polyphenols Recovery from Grape Pomace: A Comparison of Pressurized and Atmospheric Extractions with Deep Eutectic Solvent Aqueous Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Analytic Reagents
2.2. Preparation of Extraction Solvents
2.3. Grape Pomace
2.4. Atmospheric Solid-Liquid Extraction of Carménère Pomace
2.5. Hot Pressurized Liquid Extraction of Carménère Pomace
2.6. Total Polyphenol Content (TPC)
2.7. Antioxidant Capacity
2.8. Target Polyphenols Quantification
2.9. Scanning Electron Microscope (SEM) Analysis
2.10. Statistical Analysis
2.11. Quantum Chemical Calculations
3. Results
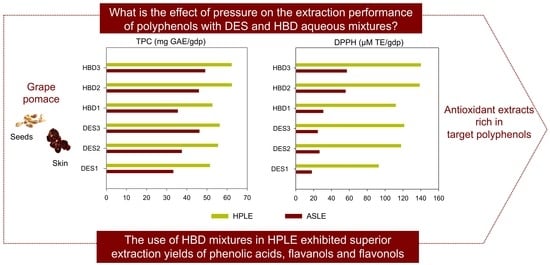
3.1. Effect of Aqueous DES and Aqueous HBD Precursors on the Extraction of TPC under ASLE and HPLE Conditions
3.2. Effect of Aqueous DES and Aqueous HBD Precursors on the Antioxidant Capacity of Carménère Pomace Extracts Obtained under ASLE and HPLE Conditions
3.3. Effect of Aqueous DES and Aqueous HBD Precursors Target Polyphenols of Carménère Pomace Extracts Obtained under ASLE and HPLE Conditions
3.3.1. Phenolic Acids
3.3.2. Flavanols
3.3.3. Flavonols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández, K.; Kennedy, J.A.; Agosin, E. Characterization of Vitis vinifera L. Cv. Carménère Grape and Wine Proanthocyanidins. J. Agric. Food Chem. 2007, 55, 3675–3680. [Google Scholar] [CrossRef]
- Huaman-Castilla, N.L.; Mariotti-Celis, M.S.; Perez-Correa, J.R. Polyphenols of Carménère Grapes. Mini Rev. Org. Chem. 2017, 14, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic Content and in Vitro Antioxidant Characteristics of Wine Industry and Other Agri-Food Solid Waste Extracts. J. Food Compos. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current Trends and Possibilities for Exploitation of Grape Pomace as a Potential Source for Value Addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef] [PubMed]
- Martínez Salgado, M.M.; Ortega Blu, R.; Janssens, M.; Fincheira, P. Grape Pomace Compost as a Source of Organic Matter: Evolution of Quality Parameters to Evaluate Maturity and Stability. J. Clean. Prod. 2019, 216, 56–63. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H. Bin Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [Green Version]
- Mena, P.; Calani, L.; Bruni, R.; Del Rio, D. Bioactivation of High-Molecular-Weight Polyphenols by the Gut Microbiome. In Diet-Microbe Interactions in the Gut: Effects on Human Health and Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 73–101. ISBN 9780124079410. [Google Scholar]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, 1–10. [Google Scholar] [CrossRef]
- Victor, A.; David, A.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction Optimisation Using Water/Glycerol for the Efficient Recovery of Polyphenolic Antioxidants from Two Artemisia Species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Bánvölgyi, S.; Dusza, E.; Namukwambi, F.K.; Kiss, I.; Stefanovits-Bányai, É.; Vatai, G. Optimization of Extraction of Phenolic Compounds from Tokaji Aszú Marc Using Response Surface Methodology. Prog. Agric. Eng. Sci. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Bilić, M.; Velić, D. Study of Solid-Liquid Extraction Kinetics of Total Polyphenols from Grape Seeds. J. Food Eng. 2007, 81, 236–242. [Google Scholar] [CrossRef]
- Castellanos-Gallo, L.; Ballinas-Casarrubias, L.; Espinoza-Hicks, J.C.; Hernández-Ochoa, L.R.; Muñoz-Castellanos, L.N.; Zermeño-Ortega, M.R.; Borrego-Loya, A.; Salas, E. Grape Pomace Valorization by Extraction of Phenolic Polymeric Pigments: A Review. Processes 2022, 10, 469. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green Alternative Methods for the Extraction of Antioxidant Bioactive Compounds from Winery Wastes and By-Products: A Review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Pérez-Correa, J.R. Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations. Biomolecules 2020, 10, 474. [Google Scholar] [CrossRef] [Green Version]
- Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Huamán-Castilla, N.; Pedreschi, F.; Iglesias-Rebolledo, N.; Pérez-Correa, J.R. Impact of an Integrated Process of Hot Pressurised Liquid Extraction–Macroporous Resin Purification over the Polyphenols, Hydroxymethylfurfural and Reducing Sugars Content of Vitis vinifera ‘Carménère’ Pomace Extracts. Int. J. Food Sci. Technol. 2018, 53, 1072–1078. [Google Scholar] [CrossRef]
- Allcca-Alca, E.E.; León-Calvo, N.C.; Luque-Vilca, O.M.; Martínez-Cifuentes, M.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Huamán-Castilla, N.L. Hot Pressurized Liquid Extraction of Polyphenols from the Skin and Seeds of Vitis vinifera L. Cv. Negra Criolla Pomace a Peruvian Native Pisco Industry Waste. Agronomy 2021, 11, 866. [Google Scholar] [CrossRef]
- Huaman-Castilla, N.L.; Martínez-Cifuentes, M.; Camilo, C.; Pedreschi, F.; Mariotti-Celis, M.; Pérez-Correa, J.R. The Impact of Temperature and Ethanol Concentration on the Global Recovery of Specific Polyphenols in an Integrated HPLE/RP Process on Carménère Pomace Extracts. Molecules 2019, 24, 3145. [Google Scholar] [CrossRef] [Green Version]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Ferreira-Santos, P.; Alfonso, I.M.; Martínez, S.; Genisheva, Z.; Gullón, B. Deep Eutectic Solvents as a Green Tool for the Extraction of Bioactive Phenolic Compounds from Avocado Peels. Molecules 2022, 27, 6646. [Google Scholar] [CrossRef]
- Khan, A.S.; Ibrahim, T.H.; Jabbar, N.A.; Khamis, M.I.; Nancarrow, P.; Mjalli, F.S. Ionic Liquids and Deep Eutectic Solvents for the Recovery of Phenolic Compounds: Effect of Ionic Liquids Structure and Process Parameters. RSC Adv. 2021, 11, 12398–12422. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Dietz, C.H.J.T.; Van Spronsen, J.; Kroon, M.C.; Gallucci, F.; Van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of Natural Deep Eutectic Solvents to the Extraction of Anthocyanins from Catharanthus Roseus with High Extractability and Stability Replacing Conventional Organic Solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef] [Green Version]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel Lactic Acid-Based Natural Deep Eutectic Solvents: Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Common Native Greek Medicinal Plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- AliBaba. 70% 75% Choline Chloride Feed Grade CAS 67-48-1. Available online: https://www.alibaba.com/product-detail/70-75-Choline-Chloride-Feed-Grade_1600863182619.html?spm=a2700.7735675.0.0.45cfcAKfcAKfm9&s=p (accessed on 5 July 2023).
- Made in China. Manufacturers Price of Organic Vegetable Refined 99.7% USP Food Grade Liquid Industrial Glycerine Glycerol. Available online: https://dd226cefa9a803c1.en.made-in-china.com/product/gmpYfFRZHUVd/China-Manufacturers-Price-of-Organic-Vegetable-Refined-99-7-USP-Food-Grade-Liquid-Industrial-Glycerine-Glycerol.html (accessed on 5 July 2023).
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Zhang, J.; Wang, Q.; Wang, F.; Qiao, Y.; Zhang, Y. Rapid Determination of Ten Polyphenols in Kudiezi Injection Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry in Multiple Reaction Monitoring Mode. Anal. Methods 2012, 4, 4230–4236. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, G.E.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, UK, 2009.
- Boys, S.F.; Bernardi, F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Řezáč, J.; Hobza, P. Benchmark Calculations of Interaction Energies in Noncovalent Complexes and Their Applications. Chem. Rev. 2016, 116, 5038–5071. [Google Scholar] [CrossRef] [PubMed]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass Valoriz. 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Florindo, C.; McIntosh, A.J.S.; Welton, T.; Branco, L.C.; Marrucho, I.M. A Closer Look into Deep Eutectic Solvents: Exploring Intermolecular Interactions Using Solvatochromic Probes. PCCP 2017, 20, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic Parameters for Solvents of Interest in Green Chemistry. Green. Chem. 2012, 14, 1245–1259. [Google Scholar] [CrossRef]
- Pal, S.; Roy, R.; Paul, S. Potential of a Natural Deep Eutectic Solvent, Glyceline, in the Thermal Stability of the Trp-Cage Mini-Protein. J. Phys. Chem. B 2020, 124, 7598–7610. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of Polyphenolic Antioxidants from Orange Peel Waste Using Deep Eutectic Solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Gajardo-Parra, N.F.; Do, H.T.; Yang, M.; Pérez-Correa, J.R.; Garrido, J.M.; Sadowski, G.; Held, C.; Canales, R.I. Impact of Deep Eutectic Solvents and Their Constituents on the Aqueous Solubility of Phloroglucinol Dihydrate. J. Mol. Liq. 2021, 344, 117932. [Google Scholar] [CrossRef]
- Tolmachev, D.; Lukasheva, N.; Ramazanov, R.; Nazarychev, V.; Borzdun, N.; Volgin, I.; Andreeva, M.; Glova, A.; Melnikova, S.; Dobrovskiy, A.; et al. Computer Simulations of Deep Eutectic Solvents: Challenges, Solutions, and Perspectives. Int. J. Mol. Sci. 2022, 23, 645. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Salinas, J.R.; Bulnes, P.; Zúñiga, M.C.; Pérez-Jiménez, J.; Torres, J.L.; Mateos-Martín, M.L.; Agosin, E.; Pérez-Correa, J.R. Effect of Pressurized Hot Water Extraction on Antioxidants from Grape Pomace before and after Enological Fermentation. J. Agric. Food Chem. 2013, 61, 6929–6936. [Google Scholar] [CrossRef]
- Otero-Pareja, M.J.; Casas, L.; Fernández-Ponce, M.T.; Mantell, C.; De La Ossa, E.J.M. Green Extraction of Antioxidants from Different Varieties of Red Grape Pomace. Molecules 2015, 20, 9686–9702. [Google Scholar] [CrossRef] [Green Version]
- Zujko, M.E.; Witkowska, A.M. Antioxidant Potential and Polyphenol Content of Selected Food. Int. J. Food Prop. 2011, 14, 300–308. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huamán-Castilla, N.L.; Campos, D.; García-Ríos, D.; Parada, J.; Martínez-Cifuentes, M.; Mariotti-Celis, M.S.; Pérez-Correa, J.R. Chemical Properties of Vitis vinifera Carménère Pomace Extracts Obtained by Hot Pressurized Liquid Extraction, and Their Inhibitory Effect on Type 2 Diabetes Mellitus Related Enzymes. Antioxidants 2021, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH Assay Comparison to Assess Antioxidant Capacity of Tea Infusions: Relationship between Total Polyphenol and Individual Catechin Content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Turner, C. Pressurized Hot Water Extraction of Bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Passos, C.P.; Cardoso, S.M.; Domingues, M.R.M.; Domingues, P.; Silva, C.M.; Coimbra, M.A. Evidence for Galloylated Type-A Procyanidins in Grape Seeds. Food Chem. 2007, 105, 1457–1467. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]

| Extraction Solvent | Co-Solvent Description | Mass Fraction | |||
|---|---|---|---|---|---|
| HBA | HBD | Water | HBA | HBD | |
| DES1 | Choline chloride | Levulinic Acid | 0.5 | 0.19 | 0.31 |
| DES2 | Choline chloride | Ethylene glycol | 0.5 | 0.25 | 0.24 |
| DES3 | Choline chloride | Glycerol | 0.5 | 0.22 | 0.28 |
| HBD1 | - | Levulinic Acid | 0.5 | - | 0.5 |
| HBD2 | - | Ethylene glycol | 0.5 | - | 0.5 |
| HBD3 | - | Glycerol | 0.5 | - | 0.5 |
| Description | TPC (mg GAE/gdp) | |
|---|---|---|
| ASLE | HPLE | |
| DES1 | 33.39 a ± 0.59 | 51.62 a ± 1.53 |
| DES2 | 37.62 a,b ± 1.03 | 55.60 b ± 0.89 |
| DES3 | 46.39 c ± 0.79 | 56.41 b ± 1.33 |
| HBD1 | 35.63 a ± 2.02 | 52.80 a ± 2.13 |
| HBD2 | 46.11 c ± 1.55 | 62.44 c ± 1.67 |
| HBD3 | 49.22 c ± 1.13 | 62.38 c ± 2.13 |
| Complex | Binding Energy (kcal/mol) |
|---|---|
| DES1 | −15.4 |
| DES2 | −19.1 |
| DES3 | −17.1 |
| Choline chloride:water | −37.4 |
| Description | DPPH µM TE/gdp | |
|---|---|---|
| ASLE | HPLE | |
| DES1 | 18.32 a ± 1.63 | 93.17 a ± 2.54 |
| DES2 | 26.90 b ± 3.18 | 118.18 c ± 3.84 |
| DES3 | 24.96 b ± 2.01 | 121.73 c ± 2.89 |
| HBD1 | 31.12 c ± 2.63 | 112.24 b ± 3.11 |
| HBD2 | 56.12 d ± 1.88 | 139.02 c,d ± 4.85 |
| HBD3 | 57.48 d ± 2.05 | 140.39 d ± 3.72 |
| Description | DES 1 | DES 2 | DES 3 | HBD 1 | HBD 2 | HBD 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASLE | HPLE | ASLE | HPLE | ASLE | HPLE | ASLE | HPLE | ASLE | HPLE | ASLE | HPLE | |
| Phenolic acids (µg/gdp) | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| Gallic | 0.01 ± 0.01 | 0.17 ± 0.01 | 0.14 ± 0.01 | 0.24 ± 0.01 | 0.17 ± 0.01 | 0.26 ± 0.02 | 1.26 ± 0.05 | 1.45 ± 0.06 | 1.40 ± 0.05 | 2.56 ± 0.14 | 1.17 ± 0.02 | 2.44c ± 0.33 |
| Caffeic | 0.01 ± 0.01 | 0.05 ± 0.00 | 0.01 ± 0.00 | 0.05 ± 0.00 | 0.02 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.10 ± 0.02 | 0.05 ± 0.01 | 0.07 ± 0.01 |
| Chlorogenic | nd | nd | nd | nd | 0.04 ± 0.01 | nd | 0.03 ± 0.01 | nd | 0.08 ± 0.01 | nd | 0.04 ± 0.01 | |
| Ʃ: | 0.02 ± 0.01 | 0.22 ± 0.01 | 0.15 ± 0.02 | 0.29 ± 0.01 | 0.19 ± 0.01 | 0.38 ± 0.02 | 1.31 ± 0.04 | 1.53 ± 0.05 | 1.47 ± 0.03 | 2.74 ± 0.08 | 1.22 ± 0.02 | 2.55 ± 0.16 |
| Flavanols (µg/gdp) | ||||||||||||
| Epigallocatechin | 0.12 ± 0.01 | 0.15 ± 0.04 | 0.10 ± 0.01 | 0.18 ± 0.01 | 0.13 ± 0.00 | 0.24 ± 0.05 | 7.92 ± 0.74 | 16.28 ± 1.29 | 11.33 ± 0.96 | 16.83 ± 1.58 | 11.95 ± 0.62 | 22.43 ± 2.39 |
| Catechin | 0.05 ± 0.01 | 0.08 ± 0.02 | 0.05 ± 0.01 | 0.09 ± 0.01 | 0.06 ± 0.02 | 0.17 ± 0.01 | 3.65 ± 0.11 | 5.76 ± 0.21 | 4.23 ± 0.13 | 6.23 ± 0.39 | 2.67 ± 0.19 | 6.01 ± 0.37 |
| Epicatechin | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.12 ± 0.02 | 0.13 ± 0.01 | 0.18 ± 0.01 | 6.74 ± 0.02 | 7.68 ± 0.42 | 8.04 ± 0.51 | 10.78 ± 1.00 | 5.82 ± 0.28 | 10.12 ± 0.64 |
| Ʃ: | 0.29 ± 0.01 | 0.34 ± 0.03 | 0.25 ± 0.01 | 0.39 ± 0.01 | 0.32 ± 0.01 | 0.59 ± 0.03 | 18.31 ± 0.44 | 29.72 ± 0.98 | 23.60 ± 0.42 | 33.84 ± 0.78 | 20.44 ± 0.29 | 38.56 ± 0.21 |
| Flavonols (µg/gdp) | ||||||||||||
| Quercetin | nd | 5.49 ± 1.35 | nd | nd | 2.23 ± 0.48 | 1.42 ± 0.19 | 5.30 ± 1.22 | 10.91 ± 0.34 | 9.46 ± 0.83 | 13.69 ± 0.91 | 3.95 ± 0.06 | 8.98 ± 0.78 |
| Kaempferol | nd | 0.47 ± 0.07 | 0.45 ± 0.08 | 1.61 ± 0.11 | nd | 5.39 ± 0.60 | 0.61 ± 0.06 | 1.49 ± 0.05 | 0.97 ± 0.18 | 5.19 ± 0.32 | 0.40 ± 0.02 | 1.31 ± 0.10 |
| Ʃ: | 5.96 ± 0.72 | 0.45 ± 0.08 | 1.61 ± 0.11 | 2.23 ± 0.48 | 6.81 ± 0.43 | 5.91 ± 0.83 | 11.40 ± 0.17 | 10.43 ± 0.39 | 18.88 ± 0.56 | 4.35 ± 0.03 | 10.29 ± 0.43 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huamán-Castilla, N.L.; Gajardo-Parra, N.; Pérez-Correa, J.R.; Canales, R.I.; Martínez-Cifuentes, M.; Contreras-Contreras, G.; Mariotti-Celis, M.S. Enhanced Polyphenols Recovery from Grape Pomace: A Comparison of Pressurized and Atmospheric Extractions with Deep Eutectic Solvent Aqueous Mixtures. Antioxidants 2023, 12, 1446. https://doi.org/10.3390/antiox12071446
Huamán-Castilla NL, Gajardo-Parra N, Pérez-Correa JR, Canales RI, Martínez-Cifuentes M, Contreras-Contreras G, Mariotti-Celis MS. Enhanced Polyphenols Recovery from Grape Pomace: A Comparison of Pressurized and Atmospheric Extractions with Deep Eutectic Solvent Aqueous Mixtures. Antioxidants. 2023; 12(7):1446. https://doi.org/10.3390/antiox12071446
Chicago/Turabian StyleHuamán-Castilla, Nils Leander, Nicolás Gajardo-Parra, José R. Pérez-Correa, Roberto I. Canales, Maximiliano Martínez-Cifuentes, Gabriela Contreras-Contreras, and María Salomé Mariotti-Celis. 2023. "Enhanced Polyphenols Recovery from Grape Pomace: A Comparison of Pressurized and Atmospheric Extractions with Deep Eutectic Solvent Aqueous Mixtures" Antioxidants 12, no. 7: 1446. https://doi.org/10.3390/antiox12071446
APA StyleHuamán-Castilla, N. L., Gajardo-Parra, N., Pérez-Correa, J. R., Canales, R. I., Martínez-Cifuentes, M., Contreras-Contreras, G., & Mariotti-Celis, M. S. (2023). Enhanced Polyphenols Recovery from Grape Pomace: A Comparison of Pressurized and Atmospheric Extractions with Deep Eutectic Solvent Aqueous Mixtures. Antioxidants, 12(7), 1446. https://doi.org/10.3390/antiox12071446