The Role of Lifestyle and Dietary Factors in the Development of Premature Ovarian Insufficiency
Abstract
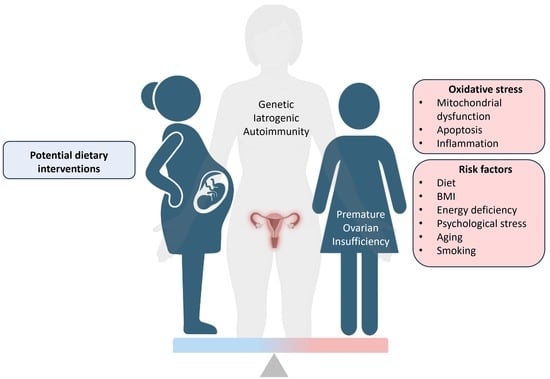
:1. Introduction
2. Hallmarks of POI
2.1. Genetic Causes of POI
2.2. Autoimmunity and the Gut Microbiota in POI
2.3. Iatrogenic POI: Chemotherapy and Radiotherapy
3. Oxidative Stress Related Mechanisms in Ovarian Ageing
3.1. Mitochondrial Dysfunction
3.2. ROS Apoptosis and Inflammation
3.3. Oestrogen and Oxidative Stress
4. Risk Factors for Primary Ovarian Insufficiency
4.1. Diet
4.2. High Fat Diet
4.3. BMI
4.4. Exercise
4.5. Other Lifestyle Factors
4.5.1. Smoking
4.5.2. Psychological Stress
4.5.3. Ageing
5. Potential Dietary Interventions
5.1. Melatonin
5.2. Mitochondria-Targeted Nutrient Therapy
5.3. Oily Fish
5.4. Dairy Consumption
5.5. Vitamin C and E
6. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cano, A. Menopause: A Comprehensive Approach; Springer: Cham, Switzerland, 2017. [Google Scholar]
- De Vos, M.; Devroey, P.; Fauser, B.C. Primary ovarian insufficiency. Lancet 2010, 376, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Shelling, A.N. Premature ovarian failure. Reproduction 2010, 140, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenton, A.J. Premature ovarian insufficiency: Pathogenesis and management. J. Mid-Life Health 2015, 6, 147. [Google Scholar] [CrossRef]
- Silvén, H.; Savukoski, S.; Pesonen, P.; Pukkala, E.; Ojaniemi, M.; Gissler, M.; Suvanto, E.; Niinimäki, M. Association of genetic disorders and congenital malformations with premature ovarian insufficiency: A nationwide register-based study. Hum. Reprod. 2023, 38, 1224–1230. [Google Scholar] [CrossRef]
- Mauri, D.; Gazouli, I.; Zarkavelis, G.; Papadaki, A.; Mavroeidis, L.; Gkoura, S.; Ntellas, P.; Amylidi, A.-L.; Tsali, L.; Kampletsas, E. Chemotherapy associated ovarian failure. Front. Endocrinol. 2020, 11, 572388. [Google Scholar] [CrossRef]
- Tiosano, D.; Mears, J.A.; Buchner, D.A. Mitochondrial dysfunction in primary ovarian insufficiency. Endocrinology 2019, 160, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.; Cree, L.; Shelling, A.N. The genetics of premature ovarian failure: Current perspectives. Int. J. Women’s Health 2015, 7, 799–810. [Google Scholar]
- Pu, D.; Xing, Y.; Gao, Y.; Gu, L.; Wu, J. Gene variation and premature ovarian failure: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 226–237. [Google Scholar] [CrossRef]
- Eshre Guideline Group on POI; Webber, L.; Davies, M.; Anderson, R.; Bartlett, J.; Braat, D.; Cartwright, B.; Cifkova, R.; de Muinck Keizer-Schrama, S.; Hogervorst, E. ESHRE Guideline: Management of women with premature ovarian insufficiency. Hum. Reprod. 2016, 31, 926–937. [Google Scholar]
- Dixit, H.; Rao, L.; Padmalatha, V.; Raseswari, T.; Kapu, A.K.; Panda, B.; Murthy, K.; Tosh, D.; Nallari, P.; Deenadayal, M. Genes governing premature ovarian failure. Reprod. Biomed. Online 2010, 20, 724–740. [Google Scholar] [CrossRef] [Green Version]
- França, M.M.; Mendonca, B.B. Genetics of ovarian insufficiency and defects of folliculogenesis. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101594. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, H.; Zhang, C. Selected Genetic Factors Associated with Primary Ovarian Insufficiency. Int. J. Mol. Sci. 2023, 24, 4423. [Google Scholar] [CrossRef]
- Louwers, Y.V.; Visser, J.A. Shared genetics between age at menopause, early menopause, POI and other traits. Front. Genet. 2021, 12, 1889. [Google Scholar] [CrossRef]
- Sharif, K.; Watad, A.; Bridgewood, C.; Kanduc, D.; Amital, H.; Shoenfeld, Y. Insights into the autoimmune aspect of premature ovarian insufficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101323. [Google Scholar] [CrossRef]
- Domniz, N.; Meirow, D. Premature ovarian insufficiency and autoimmune diseases. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 60, 42–55. [Google Scholar] [CrossRef]
- Szeliga, A.; Calik-Ksepka, A.; Maciejewska-Jeske, M.; Grymowicz, M.; Smolarczyk, K.; Kostrzak, A.; Smolarczyk, R.; Rudnicka, E.; Meczekalski, B. Autoimmune diseases in patients with premature ovarian insufficiency—Our current state of knowledge. Int. J. Mol. Sci. 2021, 22, 2594. [Google Scholar] [CrossRef]
- Kirshenbaum, M.; Orvieto, R. Premature ovarian insufficiency (POI) and autoimmunity-an update appraisal. J. Assist. Reprod. Genet. 2019, 36, 2207–2215. [Google Scholar] [CrossRef]
- Dorjgochoo, T.; Kallianpur, A.; Gao, Y.-T.; Cai, H.; Yang, G.; Li, H.; Zheng, W.; Shu, X.O. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women’s Health Study. Menopause 2008, 15, 924. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, L.-D.; Li, H. The gut microbiota: Emerging evidence in autoimmune diseases. Trends Mol. Med. 2020, 26, 862–873. [Google Scholar] [CrossRef]
- Wu, J.; Zhuo, Y.; Liu, Y.; Chen, Y.; Ning, Y.; Yao, J. Association between premature ovarian insufficiency and gut microbiota. BMC Pregnancy Childbirth 2021, 21, 418. [Google Scholar] [CrossRef]
- Jiang, L.; Fei, H.; Tong, J.; Zhou, J.; Zhu, J.; Jin, X.; Shi, Z.; Zhou, Y.; Ma, X.; Yu, H. Hormone replacement therapy reverses gut microbiome and serum metabolome alterations in premature ovarian insufficiency. Front. Endocrinol. 2021, 12, 794496. [Google Scholar] [CrossRef]
- Vieira-Silva, S.; Falony, G.; Belda, E.; Nielsen, T.; Aron-Wisnewsky, J.; Chakaroun, R.; Forslund, S.K.; Assmann, K.; Valles-Colomer, M.; Nguyen, T.T.D. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 2020, 581, 310–315. [Google Scholar] [CrossRef]
- He, S.; Li, H.; Yu, Z.; Zhang, F.; Liang, S.; Liu, H.; Chen, H.; Lü, M. The gut microbiome and sex hormone-related diseases. Front. Microbiol. 2021, 12, 2699. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef] [Green Version]
- Chhabra, S.; Kutchi, I. Fertility preservation in gynecological cancers. Clin. Med. Insights Reprod. Health 2013, 7, CMRH-S10794. [Google Scholar] [CrossRef] [Green Version]
- Podfigurna, A.; Czyzyk, A.; Grymowicz, M.; Smolarczyk, R.; Meczekalski, B. Primary ovarian insufficiency. In Menopause: A Comprehensive Approach; Springer: Cham, Switzerland, 2017; pp. 23–66. [Google Scholar]
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The role of oxidative stress in ovarian aging: A review. J. Ovarian Res. 2022, 15, 100. [Google Scholar] [CrossRef]
- May-Panloup, P.; Boucret, L.; Chao de la Barca, J.-M.; Desquiret-Dumas, V.; Ferre-L’Hotellier, V.; Moriniere, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Pathak, D.; Kriplani, A.; Ammini, A.; Talwar, P.; Dada, R. Nucleotide variations in mitochondrial DNA and supra-physiological ROS levels in cytogenetically normal cases of premature ovarian insufficiency. Arch. Gynecol. Obstet. 2010, 282, 695–705. [Google Scholar] [CrossRef]
- Ruder, E.H.; Hartman, T.J.; Goldman, M.B. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009, 21, 219. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget 2017, 8, 25310. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wu, X.Q.; Lu, S.; Guo, Y.L.; Ma, X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 2006, 16, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.-T.; Ren, Z.; Yeung, W.S.; Shu, Y.-M.; Xu, Y.-W.; Zhuang, G.-L.; Liang, X.-Y. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum. Reprod. 2007, 22, 1681–1686. [Google Scholar] [CrossRef] [Green Version]
- May-Panloup, P.; Chretien, M.; Jacques, C.; Vasseur, C.; Malthiery, Y.; Reynier, P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum. Reprod. 2005, 20, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Almeida, C.P.; Ferreira, M.C.; Silveira, C.O.; Campos, J.R.; Borges, I.T.; Baeta, P.G.; Silva, F.H.; Reis, F.M.; Del Puerto, H.L. Clinical correlation of apoptosis in human granulosa cells—A review. Cell Biol. Int. 2018, 42, 1276–1281. [Google Scholar] [CrossRef]
- Matsuda-Minehata, F.; Inoue, N.; Goto, Y.; Manabe, N. The regulation of ovarian granulosa cell death by pro-and anti-apoptotic molecules. J. Reprod. Dev. 2006, 52, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Regan, S.L.; Knight, P.G.; Yovich, J.L.; Stanger, J.D.; Leung, Y.; Arfuso, F.; Almahbobi, G.; Dharmarajan, A. The effect of ovarian reserve and receptor signalling on granulosa cell apoptosis during human follicle development. Mol. Cell. Endocrinol. 2018, 470, 219–227. [Google Scholar] [CrossRef]
- Nakahara, K.; Saito, H.; Saito, T.; Ito, M.; Ohta, N.; Takahashi, T.; Hiroi, M. The incidence of apoptotic bodies in membrana granulosa can predict prognosis of ova from patients participating in in vitro fertilization programs. Fertil. Steril. 1997, 68, 312–317. [Google Scholar] [CrossRef]
- Oosterhuis, G.J.E.; Michgelsen, H.W.; Lambalk, C.B.; Schoemaker, J.; Vermes, I. Apoptotic cell death in human granulosa-lutein cells: A possible indicator of in vitro fertilization outcome. Fertil. Steril. 1998, 70, 747–749. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The role of oxidative stress and natural antioxidants in ovarian aging. Front. Pharmacol. 2021, 11, 617843. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Liu, Y.; Liao, X.; Wu, D.; Chen, Y.; Liang, Z.; Yuan, Z.; Li, R.; Yi, J. Tannic acid repair of zearalenone-induced damage by regulating the death receptor and mitochondrial apoptosis signaling pathway in mice. Environ. Pollut. 2021, 287, 117557. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, W.; Wang, X.; Cai, P.; Jia, Q.; Zhao, W. Cell-free DNA induced apoptosis of granulosa cells by oxidative stress. Clin. Chim. Acta 2017, 473, 213–217. [Google Scholar] [CrossRef]
- Chaudhary, G.R.; Yadav, P.K.; Yadav, A.K.; Tiwari, M.; Gupta, A.; Sharma, A.; Pandey, A.N.; Pandey, A.K.; Chaube, S.K. Necroptosis in stressed ovary. J. Biomed. Sci. 2019, 26, 11. [Google Scholar] [CrossRef] [Green Version]
- Borrás, C.; Gambini, J.; Gómez-Cabrera, M.C.; Sastre, J.; Pallardó, F.V.; Mann, G.E.; Viña, J. 17β-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2 [MAPK]/NFκB cascade. Aging Cell 2005, 4, 113–118. [Google Scholar] [CrossRef]
- Ruiz-Larrea, M.B.; Martin, C.; Martinez, R.; Navarro, R.; Lacort, M.; Miller, N.J. Antioxidant activities of estrogens against aqueous and lipophilic radicals; differences between phenol and catechol estrogens. Chem. Phys. Lipids 2000, 105, 179–188. [Google Scholar] [CrossRef]
- Yoshino, K.; Komura, S.; Watanabe, I.; Nakagawa, Y.; Yagi, K. Effect of estrogens on serum and liver lipid peroxide levels in mice. J. Clin. Biochem. Nutr. 1987, 3, 233–240. [Google Scholar] [CrossRef]
- Keaney, J.F., Jr.; Shwaery, G.T.; Xu, A.; Nicolosi, R.J.; Loscalzo, J.; Foxall, T.L.; Vita, J.A. 17 beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation 1994, 89, 2251–2259. [Google Scholar] [CrossRef] [Green Version]
- Viña, J.; Gambini, J.; García-García, F.J.; Rodriguez-Mañas, L.; Borrás, C. Role of oestrogens on oxidative stress and inflammation in ageing. Horm. Mol. Biol. Clin. Investig. 2013, 16, 65–72. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Gaskins, A.J.; Mumford, S.L.; Browne, R.W.; Yeung, E.; Trevisan, M.; Hediger, M.; Zhang, C.; Perkins, N.J.; Hovey, K. Influence of endogenous reproductive hormones on F2-isoprostane levels in premenopausal women: The BioCycle Study. Am. J. Epidemiol. 2010, 172, 430–439. [Google Scholar] [CrossRef]
- Mumford, S.L.; Browne, R.W.; Schliep, K.C.; Schmelzer, J.; Plowden, T.C.; Michels, K.A.; Sjaarda, L.A.; Zarek, S.M.; Perkins, N.J.; Messer, L.C. Serum antioxidants are associated with serum reproductive hormones and ovulation among healthy women. J. Nutr. 2016, 146, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Estradiol. National Center for Biotechnology Information: PubChem Compound Summary for CID 5757. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5757 (accessed on 4 July 2023).
- Grisotto, G.; Farago, J.S.; Taneri, P.E.; Wehrli, F.; Roa-Díaz, Z.M.; Minder, B.; Glisic, M.; Gonzalez-Jaramillo, V.; Voortman, T.; Marques-Vidal, P. Dietary factors and onset of natural menopause: A systematic review and meta-analysis. Maturitas 2022, 159, 15–32. [Google Scholar] [CrossRef]
- Nagata, C.; Takatsuka, N.; Inaba, S.; Kawakami, N.; Shimizu, H. Association of diet and other lifestyle with onset of menopause in Japanese women. Maturitas 1998, 29, 105–113. [Google Scholar] [CrossRef]
- Nagel, G.; Altenburg, H.-P.; Nieters, A.; Boffetta, P.; Linseisen, J. Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas 2005, 52, 337–347. [Google Scholar] [CrossRef]
- Morris, D.H.; Jones, M.E.; Schoemaker, M.J.; McFadden, E.; Ashworth, A.; Swerdlow, A.J. Body mass index, exercise, and other lifestyle factors in relation to age at natural menopause: Analyses from the breakthrough generations study. Am. J. Epidemiol. 2012, 175, 998–1005. [Google Scholar] [CrossRef] [Green Version]
- Grisotto, G.; Langton, C.R.; Li, Y.; Bertone-Johnson, E.R.; Baden, M.Y.; Franco, O.H.; Hu, F.B.; Muka, T.; Eliassen, A.H. Association of plant-based diet and early onset of natural menopause. Menopause 2022, 29, 861. [Google Scholar] [CrossRef]
- Dunneram, Y.; Greenwood, D.C.; Burley, V.J.; Cade, J.E. Dietary intake and age at natural menopause: Results from the UK Women’s Cohort Study. J. Epidemiol. Community Health 2018, 72, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Boutot, M.E.; Purdue-Smithe, A.; Whitcomb, B.W.; Szegda, K.L.; Manson, J.E.; Hankinson, S.E.; Rosner, B.A.; Bertone-Johnson, E.R. Dietary protein intake and early menopause in the Nurses’ Health Study II. Am. J. Epidemiol. 2018, 187, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Mostrom, M.; Evans, T.J. Phytoestrogens. In Reproductive and Developmental Toxicology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 707–722. [Google Scholar]
- Robker, R.L.; Wu, L.L.-Y.; Yang, X. Inflammatory pathways linking obesity and ovarian dysfunction. J. Reprod. Immunol. 2011, 88, 142–148. [Google Scholar] [CrossRef]
- Hohos, N.M.; Skaznik-Wikiel, M.E. High-fat diet and female fertility. Endocrinology 2017, 158, 2407–2419. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.L.-Y.; Dunning, K.R.; Yang, X.; Russell, D.L.; Lane, M.; Norman, R.J.; Robker, R.L. High-fat diet causes lipotoxicity responses in cumulus–oocyte complexes and decreased fertilization rates. Endocrinology 2010, 151, 5438–5445. [Google Scholar] [CrossRef]
- Robker, R.L.; Akison, L.K.; Bennett, B.D.; Thrupp, P.N.; Chura, L.R.; Russell, D.L.; Lane, M.; Norman, R.J. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J. Clin. Endocrinol. Metab. 2009, 94, 1533–1540. [Google Scholar] [CrossRef] [Green Version]
- Luke, B.; Brown, M.B.; Stern, J.E.; Missmer, S.A.; Fujimoto, V.Y.; Leach, R.; A SART Writing Group. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum. Reprod. 2011, 26, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Jungheim, E.S.; Schoeller, E.L.; Marquard, K.L.; Louden, E.D.; Schaffer, J.E.; Moley, K.H. Diet-induced obesity model: Abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology 2010, 151, 4039–4046. [Google Scholar] [CrossRef] [Green Version]
- Reichman, M.E.; Judd, J.T.; Taylor, P.R.; Nair, P.P.; Jones, D.; Campbell, W.S. Effect of dietary fat on length of the follicular phase of the menstrual cycle in a controlled diet setting. J. Clin. Endocrinol. Metab. 1992, 74, 1171–1175. [Google Scholar]
- Hill, P.; Garbaczewski, L.; Haley, N.; Wynder, E.L. Diet and follicular development. Am. J. Clin. Nutr. 1984, 39, 771–777. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef]
- Scagy, R. Menopause and reproductive span in rural Niugini. In Proceedings of the Annual Symposium of the Papua New Guinea Medical Society, Port Moresby, Papua New Guinea, 27–30 July 1973. [Google Scholar]
- Elias, S.G.; van Noord, P.A.; Peeters, P.H.; den Tonkelaar, I.; Grobbee, D.E. Caloric restriction reduces age at menopause: The effect of the 1944–1945 Dutch famine. Menopause 2003, 10, 399–405. [Google Scholar] [CrossRef]
- Andrisani, A.; Sabbadin, C.; Minardi, S.; Favaro, A.; Donà, G.; Bordin, L.; Ambrosini, G.; Armanini, D. Persistent amenorrhea and decreased DHEAS to cortisol ratio after recovery from anorexia nervosa. Gynecol. Endocrinol. 2017, 33, 311–314. [Google Scholar] [CrossRef]
- Szegda, K.; Whitcomb, B.W.; Purdue-Smithe, A.; Boutot, M.; Manson, J.; Hankinson, S.; Rosner, B.; Bertone-Johnson, E. Adult adiposity and risk of early menopause. Hum. Reprod. 2017, 32, 2522–2531. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Jiang, A.; Yin, L.; Li, Y.; Tao, F.; Hu, H. Body mass index and age at natural menopause: A meta-analysis. Menopause 2015, 22, 469–474. [Google Scholar] [CrossRef]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45, S116–S124. [Google Scholar] [CrossRef]
- Saraç, F.; Öztekin, K.; Çelebi, G. Early menopause association with employment, smoking, divorced marital status and low leptin levels. Gynecol. Endocrinol. 2011, 27, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Olusi, S. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int. J. Obes. 2002, 26, 1159–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, M.T.; Amin, M.N.; Uddin, M.G.; Hussain, M.S.; Sarwar, M.S.; Hossain, M.K.; Uddin, S.N.; Islam, M.S. Increased concentration of serum MDA, decreased antioxidants and altered trace elements and macro-minerals are linked to obesity among Bangladeshi population. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A chronic low-grade inflammation and its markers. Cureus 2022, 14, e22711. [Google Scholar] [PubMed]
- Ali, A.; Mehta, S.; Starck, C.; Wong, M.; O’Brien, W.J.; Haswell, C.; McNabb, W.; Rutherfurd-Markwick, K.; Ahmed Nasef, N. Effect of SunGold Kiwifruit and Vitamin C Consumption on Ameliorating Exercise-Induced Stress Response in Women. Mol. Nutr. Food Res. 2021, 65, 2001219. [Google Scholar] [CrossRef]
- Hakimi, O.; Cameron, L.-C. Effect of exercise on ovulation: A systematic review. Sports Med. 2017, 47, 1555–1567. [Google Scholar] [CrossRef]
- Gifford, R.M.; O’Leary, T.J.; Wardle, S.L.; Double, R.L.; Homer, N.Z.; Howie, A.F.; Greeves, J.P.; Anderson, R.A.; Woods, D.R.; Reynolds, R.M. Reproductive and metabolic adaptation to multistressor training in women. Am. J. Physiol.-Endocrinol. Metab. 2021, 321, E281–E291. [Google Scholar] [CrossRef]
- Maryam, M.; Mahaneem, M.; Mitra, O.M. Effect of exercise on reproductive hormones in female athletes. Int. J. Sport Exerc. Sci. 2013, 5, 7–12. [Google Scholar]
- Gudmundsdottir, S.; Flanders, W.; Augestad, L. Physical activity and age at menopause: The Nord-Trøndelag population-based health study. Climacteric 2012, 16, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Whitcomb, B.W.; Purdue-Smithe, A.C.; Manson, J.E.; Hankinson, S.E.; Rosner, B.A.; Bertone-Johnson, E.R. Physical activity is not related to risk of early menopause in a large prospective study. Hum. Reprod. 2018, 33, 1960–1967. [Google Scholar] [CrossRef] [Green Version]
- Collins, G.G.; Rossi, B.V. The impact of lifestyle modifications, diet, and vitamin supplementation on natural fertility. Fertil. Res. Pract. 2015, 1, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augood, C.; Duckitt, K.; Templeton, A. Smoking and female infertility: A systematic review and meta-analysis. Hum. Reprod. 1998, 13, 1532–1539. [Google Scholar] [CrossRef] [Green Version]
- Firns, S.; Cruzat, V.F.; Keane, K.N.; Joesbury, K.A.; Lee, A.H.; Newsholme, P.; Yovich, J.L. The effect of cigarette smoking, alcohol consumption and fruit and vegetable consumption on IVF outcomes: A review and presentation of original data. Reprod. Biol. Endocrinol. 2015, 13, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowińska-Przepiera, E.; Andrysiak-Mamos, E.; Jarząbek-Bielecka, G.; Walkowiak, A.; Osowicz-Korolonek, L.; Syrenicz, M.; Kędzia, W.; Syrenicz, A. Functional hypothalamic amenorrhoea–diagnostic challenges, monitoring, and treatment. Endokrynol. Pol. 2015, 66, 252–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, L.; Young, E.; El Khoudary, S.; Farhat, G.; Sowers, M.; Sutton-Tyrrell, K.; Newman, A. The Association of Menopause Status with Physical Function: The Study of Women’s Health Across the Nation (SWAN). Menopause 2012, 19, 1186. [Google Scholar] [CrossRef] [Green Version]
- Allshouse, A.A.; Semple, A.L.; Santoro, N.F. Evidence for prolonged and unique amenorrhea-related symptoms in women with premature ovarian failure/primary ovarian insufficiency. Menopause 2015, 22, 166–174. [Google Scholar] [CrossRef]
- Fu, X.Y.; Chen, H.H.; Zhang, N.; Ding, M.X.; Qiu, Y.E.; Pan, X.M.; Fang, Y.S.; Lin, Y.P.; Zheng, Q.; Wang, W.Q. Effects of chronic unpredictable mild stress on ovarian reserve in female rats: Feasibility analysis of a rat model of premature ovarian failure. Mol. Med. Rep. 2018, 18, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Maidarti, M.; Anderson, R.A.; Telfer, E.E. Crosstalk between PTEN/PI3K/Akt signalling and DNA damage in the oocyte: Implications for primordial follicle activation, oocyte quality and ageing. Cells 2020, 9, 200. [Google Scholar] [CrossRef] [Green Version]
- Brosens, J.J.; Bennett, P.R.; Abrahams, V.M.; Ramhorst, R.; Coomarasamy, A.; Quenby, S.; Lucas, E.S.; McCoy, R.C. Maternal selection of human embryos in early gestation: Insights from recurrent miscarriage. Semin. Cell Dev. Biol. 2022, 131, 14–24. [Google Scholar] [CrossRef]
- Rubio, C.; Rodrigo, L.; Garcia-Pascual, C.; Peinado, V.; Campos-Galindo, I.; Garcia-Herrero, S.; Simón, C. Clinical application of embryo aneuploidy testing by next-generation sequencing. Biol. Reprod. 2019, 101, 1083–1090. [Google Scholar] [CrossRef]
- Webster, A.; Schuh, M. Mechanisms of aneuploidy in human eggs. Trends Cell Biol. 2017, 27, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Wilding, M.; Dale, B.; Marino, M.; di Matteo, L.; Alviggi, C.; Pisaturo, M.L.; Lombardi, L.; De Placido, G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum. Reprod. 2001, 16, 909–917. [Google Scholar] [CrossRef]
- Han, Q.; Chen, Z.-J.; Du, Y. Dietary supplementation for female infertility: Recent advances in the nutritional therapy for premature ovarian insufficiency. Front. Microbiol. 2022, 13, 1001209. [Google Scholar] [CrossRef]
- Lin, J.; Wu, D.; Jia, L.; Liang, M.; Liu, S.; Qin, Z.; Zhang, J.; Han, Y.; Liu, S.; Zhang, Y. The treatment of complementary and alternative medicine on premature ovarian failure. Evid.-Based Complement. Altern. Med. 2021, 2021, 6677767. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, M.-Y.; Chen, Q.-C.; Xie, Y.-Y.; Li, C.-Y.; Han, F.-J. Current Research on Complementary and Alternative Medicine in the Treatment of Premature Ovarian Failure: An Update Review. Evid.-Based Complement. Altern. Med. 2022, 2022, 2574438. [Google Scholar] [CrossRef] [PubMed]
- Morvaridzadeh, M.; Sadeghi, E.; Agah, S.; Nachvak, S.M.; Fazelian, S.; Moradi, F.; Persad, E.; Heshmati, J. Effect of melatonin supplementation on oxidative stress parameters: A systematic review and meta-analysis. Pharmacol. Res. 2020, 161, 105210. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; Sugino, N. Melatonin and female reproduction. J. Obstet. Gynaecol. Res. 2014, 40, 1–11. [Google Scholar] [CrossRef]
- Brzezinski, A.; Seibel, M.M.; Lynch, H.J.; Deng, M.-H.; Wurtman, R.J. Melatonin in human preovulatory follicular fluid. J. Clin. Endocrinol. Metab. 1987, 64, 865–867. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamura, H.; Takayama, H.; Kato, H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil. Steril. 2003, 80, 1012–1016. [Google Scholar] [CrossRef]
- Takasaki, A.; Nakamura, Y.; Tamura, H.; Shimamura, K.; Morioka, H. Melatonin as a new drug for improving oocyte quality. Reprod. Med. Biol. 2003, 2, 139–144. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y. Melatonin as a free radical scavenger in the ovarian follicle. Endocr. J. 2013, 60, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batıoğlu, A.S.; Şahin, U.; Gürlek, B.; Öztürk, N.; Ünsal, E. The efficacy of melatonin administration on oocyte quality. Gynecol. Endocrinol. 2012, 28, 91–93. [Google Scholar] [CrossRef]
- Leem, J.; Bai, G.Y.; Kim, J.S.; Oh, J.S. Melatonin protects mouse oocytes from DNA damage by enhancing nonhomologous end-joining repair. J. Pineal Res. 2019, 67, e12603. [Google Scholar] [CrossRef] [PubMed]
- Barberino, R.S.; Lins, T.L.B.; Monte, A.P.; Gouveia, B.B.; Campinho, D.S.; Palheta, R.C., Jr.; Smitz, J.E.; Matos, M.H.T. Melatonin attenuates cyclophosphamide-induced primordial follicle loss by interaction with MT1 receptor and modulation of PTEN/Akt/FOXO3a proteins in the mouse ovary. Reprod. Sci. 2022, 29, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Ma, W.-W.; Li, H.-X.; Pei, X.-Y.; Deng, S.-L.; Jia, H.; Ma, W.-Z. Melatonin prevents cyclophosphamide-induced primordial follicle loss by inhibiting ovarian granulosa cell apoptosis and maintaining AMH expression. Front. Endocrinol. 2022, 13, 895095. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, M.; Guidarelli, A.; Cantoni, O. Mitochondrial reactive oxygen species: The effects of mitochondrial ascorbic acid vs. untargeted and mitochondria-targeted antioxidants. Int. J. Radiat. Biol. 2021, 97, 1055–1062. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Nabavi, S.F.; Daglia, M.; Rastrelli, L.; Nabavi, S.M. Epigallocatechin gallate and mitochondria—A story of life and death. Pharmacol. Res. 2016, 104, 70–85. [Google Scholar] [CrossRef]
- Adikesavan, G.; Vinayagam, M.M.; Abdulrahman, L.A.; Chinnasamy, T. (−)-Epigallocatechin-gallate (EGCG) stabilize the mitochondrial enzymes and inhibits the apoptosis in cigarette smoke-induced myocardial dysfunction in rats. Mol. Biol. Rep. 2013, 40, 6533–6545. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, Z.; Li, X.; Du, D.; Wu, T.; Zhou, S.; Yan, W.; Wu, M.; Jin, Y.; Zhang, J. Epigallocatechin gallate and theaflavins independently alleviate cyclophosphamide-induced ovarian damage by inhibiting the overactivation of primordial follicles and follicular atresia. Phytomedicine 2021, 92, 153752. [Google Scholar] [CrossRef]
- Huang, Z.; Pang, Y.; Hao, H.; Du, W.; Zhao, X.; Zhu, H. Effects of epigallocatechin-3-gallate on bovine oocytes matured in vitro. Asian-Australas. J. Anim. Sci. 2018, 31, 1420. [Google Scholar] [CrossRef]
- Chao, S.; Li, L.-J.; Lu, J.; Zhao, S.-X.; Zhao, M.-H.; Huang, G.-A.; Yin, S.; Shen, W.; Sun, Q.-Y.; Zhao, Y. Epigallocatechin gallate improves the quality of diabetic oocytes. Biomed. Pharmacother. 2023, 159, 114267. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wu, M.; Shao, F.; Guan, G.; Huang, B.; Tan, B.; Yin, Y. N-Acetyl-L-cysteine protects the enterocyte against oxidative damage by modulation of mitochondrial function. Mediat. Inflamm. 2016, 2016, 8364279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoldo, M.J.; Listijono, D.R.; Ho, W.-H.J.; Riepsamen, A.H.; Goss, D.M.; Richani, D.; Jin, X.L.; Mahbub, S.; Campbell, J.M.; Habibalahi, A. NAD+ repletion rescues female fertility during reproductive aging. Cell Rep. 2020, 30, 1670–1681.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, L.; Fan, L.-H.; Jing, Y.; Li, J.; Ouyang, Y.-C.; Wang, Z.-B.; Hou, Y.; Sun, Q.-Y. N-acetyl-L-cysteine (NAC) delays post-ovulatory oocyte aging in mouse. Aging 2019, 11, 2020. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.; Ye, X.; Liu, K.; Huang, J.; Wang, L.; Ji, G.; Liu, N.; Tang, X.; Baltz, J.M. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Hum. Reprod. 2012, 27, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-J.; Sun, A.-G.; Zhao, S.-G.; Liu, H.; Ma, S.-Y.; Li, M.; Huai, Y.-X.; Zhao, H.; Liu, H.-B. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil. Steril. 2018, 109, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Faga, T.; Pisani, A.; Serra, R.; Russo, D.; De Sarro, G.; Michael, A. Quercetin protects against radiocontrast medium toxicity in human renal proximal tubular cells. J. Cell. Physiol. 2018, 233, 4116–4125. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.R.; Nabavi, S.M.; Braidy, N.; Setzer, W.N.; Ahmed, T.; Nabavi, S.F. Quercetin and the mitochondria: A mechanistic view. Biotechnol. Adv. 2016, 34, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Ben-Meir, A.; Burstein, E.; Borrego-Alvarez, A.; Chong, J.; Wong, E.; Yavorska, T.; Naranian, T.; Chi, M.; Wang, Y.; Bentov, Y. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015, 14, 887–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braakhuis, A.J.; Nagulan, R.; Somerville, V. The effect of MitoQ on aging-related biomarkers: A systematic review and meta-analysis. Oxidative Med. Cell. Longev. 2018, 2018, 8575263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumegi, K.; Fekete, K.; Antus, C.; Debreceni, B.; Hocsak, E.; Gallyas, F., Jr.; Sumegi, B.; Szabo, A. BGP-15 protects against oxidative stress-or lipopolysaccharide-induced mitochondrial destabilization and reduces mitochondrial production of reactive oxygen species. PLoS ONE 2017, 12, e0169372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.L.; Russell, D.L.; Wong, S.L.; Chen, M.; Tsai, T.-S.; St John, J.C.; Norman, R.J.; Febbraio, M.A.; Carroll, J.; Robker, R.L. Mitochondrial dysfunction in oocytes of obese mothers: Transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development 2015, 142, 681–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Bispham, N.Z.; Santos-Parker, J.R.; Steward, C.A.; Cuevas, L.M.; Rosenberg, H.L.; Murphy, M.P.; Seals, D.R.; Rossman, M.J. MitoQ Supplementation Improves Leg-Extension Power in Healthy Late Middle-Aged and Older Adults. FASEB J. 2017, 31, lb852. [Google Scholar]
- Mason, S.A.; Wadley, G.D.; Keske, M.A.; Parker, L. Effect of mitochondrial-targeted antioxidants on glycaemic control, cardiovascular health, and oxidative stress in humans: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2022, 24, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; MacRae, C.L.; Broome, S.C.; D’souza, R.F.; Narang, R.; Wang, H.W.; Mori, T.A.; Hickey, A.J.; Mitchell, C.J.; Merry, T.L. MitoQ and CoQ10 supplementation mildly suppresses skeletal muscle mitochondrial hydrogen peroxide levels without impacting mitochondrial function in middle-aged men. Eur. J. Appl. Physiol. 2020, 120, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Broome, S.; Braakhuis, A.; Mitchell, C.J.; Merry, T. Mitochondria-targeted antioxidant supplementation improves 8 km time trial performance in middle-aged trained male cyclists. J. Int. Soc. Sports Nutr. 2021, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.; Hughes, C.M.; Cobley, J.N.; Davison, G.W. The mitochondria-targeted antioxidant MitoQ, attenuates exercise-induced mitochondrial DNA damage. Redox Biol. 2020, 36, 101673. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Pekas, E.J.; Headid, R.J., 3rd; Son, W.-M.; Wooden, T.K.; Song, J.; Layec, G.; Yadav, S.K.; Mishra, P.K.; Pipinos, I.I. Acute mitochondrial antioxidant intake improves endothelial function, antioxidant enzyme activity, and exercise tolerance in patients with peripheral artery disease. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H456–H467. [Google Scholar] [CrossRef] [PubMed]
- Al-Safi, Z.A.; Liu, H.; Carlson, N.E.; Chosich, J.; Harris, M.; Bradford, A.P.; Robledo, C.; Eckel, R.H.; Polotsky, A.J. Omega-3 fatty acid supplementation lowers serum FSH in normal weight but not obese women. J. Clin. Endocrinol. 2016, 101, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehra, D.; Le, H.D.; Fallon, E.M.; Carlson, S.J.; Woods, D.; White, Y.A.; Pan, A.H.; Guo, L.; Rodig, S.J.; Tilly, J.L. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell 2012, 11, 1046–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohos, N.M.; Elliott, E.M.; Cho, K.J.; Lin, I.S.; Rudolph, M.C.; Skaznik-Wikiel, M.E. High-fat diet-induced dysregulation of ovarian gene expression is restored with chronic omega-3 fatty acid supplementation. Mol. Cell. Endocrinol. 2020, 499, 110615. [Google Scholar] [CrossRef] [PubMed]
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE 2017, 12, e0187934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bo, L.; Jiang, S.; Xie, Y.; Kan, H.; Song, W.; Zhao, J. Effect of vitamin E and omega-3 fatty acids on protecting ambient PM2.5-induced inflammatory response and oxidative stress in vascular endothelial cells. PLoS ONE 2016, 11, e0152216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, B.B.; Derraik, J.G.; Cameron-Smith, D.; Hofman, P.L.; Tumanov, S.; Villas-Boas, S.G.; Garg, M.L.; Cutfield, W.S. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci. Rep. 2015, 5, 07928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, D.V.; Rani, M.U.; Reddy, A.G.; Kumar, B.K.; Reddy, M.A.; Lakshman, M.; Rajkumar, U. Protective effect of alpha-lipoic acid and omega-3 fatty acids against cyclophosphamide-induced ovarian toxicity in rats. Vet. World 2020, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Nasef, N.; Zhu, X.; Golding, M.; Dave, A.; Ali, A.; Singh, H.; Garg, G. Salmon food matrix influences digestion and bioavailability of long-chain omega-3 polyunsaturated fatty acids. Food Funct. 2021, 12, 6588–6602. [Google Scholar] [CrossRef] [PubMed]
- Purdue-Smithe, A.C.; Whitcomb, B.W.; Manson, J.E.; Hankinson, S.E.; Rosner, B.A.; Troy, L.M.; Bertone-Johnson, E.R. A prospective study of dairy-food intake and early menopause. Am. J. Epidemiol. 2019, 188, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Carwile, J.L.; Willett, W.C.; Michels, K.B. Consumption of low-fat dairy products may delay natural menopause. J. Nutr. 2013, 143, 1642–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moslehi, N.; Mirmiran, P.; Azizi, F.; Tehrani, F.R. Do dietary intakes influence the rate of decline in anti-Mullerian hormone among eumenorrheic women? A population-based prospective investigation. Nutr. J. 2019, 18, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakawa, M.; Wada, K.; Nakashima, Y.; Nagata, C. Dietary lactose and galactose intakes are associated with a later onset of natural menopause among women in a Japanese community. Br. J. Nutr. 2023, 129, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervato, G.; Cazzola, R.; Cestaro, B. Studies on the antioxidant activity of milk caseins. Int. J. Food Sci. Nutr. 1999, 50, 291–296. [Google Scholar] [PubMed]
- Zulueta, A.; Maurizi, A.; Frígola, A.; Esteve, M.; Coli, R.; Burini, G. Antioxidant capacity of cow milk, whey and deproteinized milk. Int. Dairy J. 2009, 19, 380–385. [Google Scholar] [CrossRef]
- Grażyna, C.; Hanna, C.; Adam, A.; Magdalena, B.M. Natural antioxidants in milk and dairy products. Int. J. Dairy Technol. 2017, 70, 165–178. [Google Scholar] [CrossRef]
- Kim, H.-K.; Kim, S.-R.; Ahn, J.-Y.; Cho, I.-J.; Yoon, C.-S.; Ha, T.-Y. Dietary conjugated linoleic acid reduces lipid peroxidation by increasing oxidative stability in rats. J. Nutr. Sci. Vitaminol. 2005, 51, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, Y.M.; Kadir, A.A.; Ahmad, Z.; Yaakub, H.; Zakaria, Z.A.; Hakim Abdullah, M.N. Free radical scavenging activity of conjugated linoleic acid as single or mixed isomers. Pharm. Biol. 2012, 50, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäkivuokko, H.A.; Saarinen, M.T.; Ouwehand, A.C.; Rautonen, N.E. Effects of lactose on colon microbial community structure and function in a four-stage semi-continuous culture system. Biosci. Biotechnol. Biochem. 2006, 70, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.; Darby, A.C.; Hall, N.; Nau, A.; Bravo, D.; Shirazi-Beechey, S.P. Dietary supplementation with lactose or artificial sweetener enhances swine gut Lactobacillus population abundance. Br. J. Nutr. 2014, 111, S30–S35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, T.; Venema, K.; Priebe, M.; Welling, G.; Brummer, R.J.; Vonk, R. The role of colonic metabolism in lactose intolerance. Eur. J. Clin. Investig. 2008, 38, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533. [Google Scholar] [CrossRef] [PubMed]
- Cramer, D.W.; Harlow, B.L.; Barbieri, R.L.; Ng, W.G. Galactose-1-phosphate uridyl transferase activity associated with age at menopause and reproductive history. Fertil. Steril. 1989, 51, 609–615. [Google Scholar] [CrossRef]
- Kaufman, F.R.; Kogut, M.D.; Donnell, G.N.; Goebelsmann, U.; March, C.; Koch, R. Hypergonadotropic hypogonadism in female patients with galactosemia. N. Engl. J. Med. 1981, 304, 994–998. [Google Scholar] [CrossRef]
- Cooper, G.S.; Hulka, B.S.; Baird, D.D.; Savitz, D.A.; Hughes, C.L., Jr.; Weinberg, C.R.; Coleman, R.A.; Shields, J.M. Galactose consumption, metabolism, and follicle-stimulating hormone concentrations in women of late reproductive age. Fertil. Steril. 1994, 62, 1168–1175. [Google Scholar] [CrossRef]
- Kunt, C.; Ozaksit, G.; Keskin Kurt, R.; Cakir Gungor, A.N.; Kanat-Pektas, M.; Kilic, S.; Dede, A. Anti-Mullerian hormone is a better marker than inhibin B, follicle stimulating hormone, estradiol or antral follicle count in predicting the outcome of in vitro fertilization. Arch. Gynecol. Obstet. 2011, 283, 1415–1421. [Google Scholar] [CrossRef]
- Kallio, S.; Aittomäki, K.; Piltonen, T.; Veijola, R.; Liakka, A.; Vaskivuo, T.; Dunkel, L.; Tapanainen, J. Anti-Müllerian hormone as a predictor of follicular reserve in ovarian insufficiency: Special emphasis on FSH-resistant ovaries. Hum. Reprod. 2012, 27, 854–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, J.A.; Schipper, I.; Laven, J.S.; Themmen, A.P. Anti-Müllerian hormone: An ovarian reserve marker in primary ovarian insufficiency. Nat. Rev. Endocrinol. 2012, 8, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Henmi, H.; Endo, T.; Kitajima, Y.; Manase, K.; Hata, H.; Kudo, R. Effects of ascorbic acid supplementation on serum progesterone levels in patients with a luteal phase defect. Fertil. Steril. 2003, 80, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Abdollahifar, M.-A.; Azad, N.; Sajadi, E.; Mofarahe, Z.S.; Zare, F.; Moradi, A.; Rezaee, F.; Gholamin, M.; Abdi, S. Vitamin C restores ovarian follicular reservation in a mouse model of aging. Anat. Cell Biol. 2019, 52, 196–203. [Google Scholar] [CrossRef]
- Saygin, M.; Ozmen, O.; Erol, O.; Ellidag, H.Y.; Ilhan, I.; Aslankoc, R. The impact of electromagnetic radiation (2.45 GHz, Wi-Fi) on the female reproductive system: The role of vitamin C. Toxicol. Ind. Health 2018, 34, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ding, C.; Shen, H.; Qian, C.; Zou, Q.; Lu, J.; Huang, B.; Tan, J.; Li, H. Vitamin C improves the therapeutic potential of human amniotic epithelial cells in premature ovarian insufficiency disease. Stem Cell Res. Ther. 2020, 11, 159. [Google Scholar] [CrossRef] [Green Version]
- Vandewoude, M.; Vandewoude, M.G. Vitamin E status in a normal population: The influence of age. J. Am. Coll. Nutr. 1987, 6, 307–311. [Google Scholar] [CrossRef]
- Ma, L.; Chen, G.; Xu, W.; Chen, P.; Lan, Y.; Huang, Y.; Li, C.; Zhou, J. The relationship between vitamin E level and premature ovarian insufficiency. J. Obstet. Gynaecol. Res. 2021, 47, 1481–1486. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelling, A.N.; Ahmed Nasef, N. The Role of Lifestyle and Dietary Factors in the Development of Premature Ovarian Insufficiency. Antioxidants 2023, 12, 1601. https://doi.org/10.3390/antiox12081601
Shelling AN, Ahmed Nasef N. The Role of Lifestyle and Dietary Factors in the Development of Premature Ovarian Insufficiency. Antioxidants. 2023; 12(8):1601. https://doi.org/10.3390/antiox12081601
Chicago/Turabian StyleShelling, Andrew N., and Noha Ahmed Nasef. 2023. "The Role of Lifestyle and Dietary Factors in the Development of Premature Ovarian Insufficiency" Antioxidants 12, no. 8: 1601. https://doi.org/10.3390/antiox12081601
APA StyleShelling, A. N., & Ahmed Nasef, N. (2023). The Role of Lifestyle and Dietary Factors in the Development of Premature Ovarian Insufficiency. Antioxidants, 12(8), 1601. https://doi.org/10.3390/antiox12081601