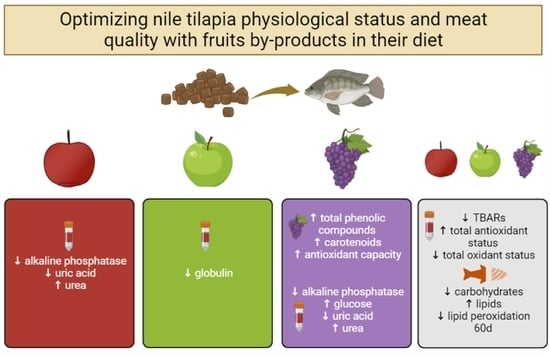
Dietary Fruit By-Products Improve the Physiological Status of Nile Tilapias (Oreochromis niloticus) and the Quality of Their Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design, Diets and Management
2.2. Determination of Bioactive Compounds and Antioxidant Activity in the Fruit Meals
2.3. Stunning, Slaughtering and Sampling Procedures
2.4. Determination of Growth Performance
2.5. Determination of the Blood Biochemical and Oxidative Parameters
2.6. Determination of the Fillets Proximate Composition and Lipid Peroxidation
2.7. Statistical Analysis
3. Results
3.1. Bioactive Compounds and Antioxidant Capacity in the Fruit By-Products
3.2. Growth Performance
3.3. Blood Biochemical and Oxidative Parameters of the Tilapias
3.4. Proximate Composition and Lipid Peroxidation in the Tilapias’ Fillets
4. Discussion
4.1. Animal Performance
4.2. Biochemical Parameters
4.3. Oxidative Status
4.4. Proximate Composition
4.5. Lipid Peroxidation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations (UN). Growing at a Slower Pace, World Population is Expected to Reach 9.7 Billion in 2050 and Could Peak at Nearly 11 Billion around 2100. New York; 2019. Available online: https://www.un.org/development/desa/en/news/population/world-population-prospects-2019.html (accessed on 14 February 2023).
- United Nations (UN). Report on the Millennium Development Goals. New York; 2015. Available online: https://sdgs.un.org/goals (accessed on 10 March 2022).
- Associação Brasileira da Piscicultura, PEIXE BR. Anuário Brasileiro da Piscicultura Peixe BR 2023; PEIXE BR: São Paulo, Brazil, 2023. [Google Scholar]
- Chikowi, C.T.; Ochieng, D.O.; Jumbe, C.B. Consumer choices and demand for Tilapia in urban Malawi: What are the complementarities and trade-offs? Aquaculture 2020, 530, 735755. [Google Scholar] [CrossRef]
- Companhia Nacional de Abastecimento (CONAB). Boletim Hortigranjeiro. 2023; Volume 9. Available online: https://www.conab.gov.br/mazonian/k2/item/download/46155_75fdc0987ae8bda1c19a7895add1ac79 (accessed on 27 January 2023).
- Abdel-Daim, M.M.; Dawood, M.A.; Elbadawy, M.; Aleya, L.; Alkahtani, S. Spirulina platensis Reduced Oxidative Damage Induced by Chlorpyrifos Toxicity in Nile Tilapia (Oreochromis niloticus). Animals 2020, 10, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georganas, A.; Giamouri, E.; Pappas, A.C.; Papadomichelakis, G.; Galliou, F.; Manios, T.; Tsiplakou, E.; Fegeros, K.; Zervas, G. Bioactive Compounds in Food Waste: A Review on the Transformation of Food Waste to Animal Feed. Foods 2020, 9, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, R.; Oliveira, H.; Fernandes, I.; Simal-Gandara, J.; Perez-Gregorio, R. Recent advances in extracting phenolic compounds from food and their use in disease prevention and as cosmetics. Crit. Rev. Food Sci. Nutr. 2020, 61, 1130–1151. [Google Scholar] [CrossRef] [PubMed]
- Kasapidou, E.; Sossidou, E.; Mitlianga, P. Fruit and Vegetable Co-Products as Functional Feed Ingredients in Farm Animal Nutrition for Improved Product Quality. Agriculture 2015, 5, 1020–1034. [Google Scholar] [CrossRef] [Green Version]
- Jurgilevich, A.; Birge, T.; Kentala-Lehtonen, J.; Korhonen-Kurki, K.; Pietikäinen, J.; Saikku, L.; Schösler, H. Transition towards Circular Economy in the Food System. Sustainability 2016, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Colombino, E.; Zduńczyk, Z.; Jankowski, J.; Cocolin, L.S.; Schiavone, A.; Biasato, I.; Prieto-Botella, D.; Karlińska, E.; Kosmala, M.; Ognik, K.; et al. Effects of Feeding Dried Fruit Pomaces as Additional Fibre-Phenolic Compound on Meat Quality, Blood Chemistry and Redox Status of Broilers. Animals 2020, 10, 1968. [Google Scholar] [CrossRef]
- Uenojo, M.; Junior, M.R.M.; Pastore, G.M. Carotenóides: Propriedades, aplicações e biotransformação para formação de compostos de aroma. Quím. Nova 2007, 30, 616–622. [Google Scholar] [CrossRef] [Green Version]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Silva, M.L.C.; Costa, R.S.; Santana, A.S.; Koblitz, M.G.B. Compostos fenólicos, carotenoides e atividade antioxidante em produtos vegetais. Semin. Cienc. Agrar. 2010, 31, 669–682. [Google Scholar] [CrossRef] [Green Version]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. J. Chem. 2016, 2016, 3164312. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Wiegertjes, G. Properties of carotenoids in fish fitness: A review. Mar. Drugs 2020, 18, 568. [Google Scholar] [CrossRef]
- García-Chavarría, M.; Flores, M.L. The use of carotenoid in aquaculture. Res. J. Fish. Hydrobiol. 2013, 8, 38–49. [Google Scholar]
- Jamshidi-Kia, F.; Wibowo, J.P.; ElAchouri, M.; Masumi, R.; Salehifard-Jouneghani, A.; Abolhasanzadeh, Z.; Lorigooini, Z. Battle between plants as antioxidants with free radicals in human body. J. Herbmed Pharmacol. 2020, 9, 191–199. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Umayaparvathi, S.; Meenakshi, S.; Vimalraj, V.; Arumugam, M.; Balasubramanian, T. Isolation and structural elucidation of antioxidant peptides from oyster (Saccostrea cucullata) protein hydrolysate. Protein Pept. Lett. 2014, 21, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Grassi, T.L.M.; Oliveira, D.L.; Paiva, N.M.; Diniz, J.C.P.; Bosco, A.M.; Pereira, A.A.F.; Menezes, A.R.P.; Valadares, T.C.; Pastor, R.C.P.; Ciarlini, P.C.; et al. Microbial biomass as an antioxidant for tilapia feed. Aquac. Res. 2018, 49, 2881–2890. [Google Scholar] [CrossRef] [Green Version]
- Mushtaq, M.; Fatima, M.; Shah, S.Z.H.; Khan, N.; Naveed, S.; Khan, M. Effects of sodium selenite, selenium methionine, and selenium yeast on growth performance, carcass composition, blood biochemistry, and antioxidant status of intensively reared Hypophthalmichthys molitrix. Aquac. Rep. 2022, 24, 101182. [Google Scholar] [CrossRef]
- Raeisi, S.; Ojagh, S.M.; Pourashouri, P.; Salaün, F.; Quek, S.Y. Shelf-life and quality of chicken nuggets fortified with encapsulated fish oil and garlic essential oil during refrigerated storage. J. Food Sci. Technol. 2021, 58, 121–128. [Google Scholar] [CrossRef]
- Yu, Q.; Xia, C.; Han, F.; Xu, C.; Rombenso, A.; Qin, J.G.; Chen, L.; Li, E. Effect of Different Dietary Selenium Sources on Growth Performance, Antioxidant Capacity, Gut Microbiota, and Molecular Responses in Pacific White Shrimp Litopenaeus vannamei. Aquac. Nutr. 2022, 2022, 5738008. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Bohrer, B.; Lorenzo, J.M. Protein Oxidation in Muscle Foods: A Comprehensive Review. Antioxidants 2022, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, A.; Sancho, A. A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Sci. 2008, 79, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.-D.A.; Hopkins, D.L.; Fahri, F.T.; Ponnampalam, E.N. Oxidative Processes in Muscle Systems and Fresh Meat: Sources, Markers, and Remedies. Compr. Rev. Food Sci. Food Saf. 2013, 12, 565–597. [Google Scholar] [CrossRef]
- Estévez, M. Critical overview of the use of plant antioxidants in the meat industry: Opportunities, innovative applications and future perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef]
- Canepelle, E.; Writzl, T.C.; Steffler, A.D.; Redin, M.; Weber, F.H.; Scherer, G.C.R.D.S. Influência dos métodos de secagem e preparo das amostras no processo de desidratação e reidratação do Abacaxi Pérola Ananas comosus L. Rev. Bras. Tecnol. Agroind. 2020, 14, 3267–3283. [Google Scholar] [CrossRef]
- Beltrán, J.M.G.; Esteban, M. Nature-identical compounds as feed additives in aquaculture. Fish Shellfish. Immunol. 2022, 123, 409–416. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Habotta, O.A.E.; Elsabagh, M.; Azra, M.N.; Van Doan, H.; Kari, Z.A.; Sewilam, H. Fruit processing by-products in the aquafeed industry: A feasible strategy for aquaculture sustainability. Rev. Aquac. 2022, 14, 1945–1965. [Google Scholar] [CrossRef]
- Caipang, C.M.A.; Omar, J.M.; Plasus, M.M.G. Plant and fruit waste products as phytogenic feed additives in aquaculture. Aquac. Aquar. Conserv. Legis. 2019, 12, 261–268. [Google Scholar]
- Hrubec, T.C.; Cardinale, J.L.; Smith, S.A. Hematology and Plasma Chemistry Reference Intervals for Cultured Tilapia (Oreochromis hybrid). Vet. Clin. Pathol. 2000, 29, 7–12. [Google Scholar] [CrossRef]
- Satheeshkumar, P.; Senthilkumar, D.; Ananthan, G.; Soundarapandian, P.; Khan, A.B. Measurement of hematological and biochemical studies on wild marine carnivorous fishes from Vellar estuary, southeast coast of India. Comp. Clin. Pathol. 2011, 20, 127–134. [Google Scholar] [CrossRef]
- Furuya, W.M.; Pezzato, L.E.; Barros, M.M.; Boscolo, W.R.; Cyrino, J.E.E.; Furuya, V.R.B.; Feiden, A. Tabelas Brasileiras para Nutrição de Tilápias; GFM: Toledo, Brazil, 2010; 100p. [Google Scholar]
- NUTRIMAX. 2022. Version 13.10 [Computer Software]. Available online: http://nutrimax.app.br (accessed on 5 April 2021).
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Effect of Drying Temperature on the Stability of Polyphenols and Antioxidant Activity of Red Grape Pomace Peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.S.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas pela Captura do Radical Livre ABTS+; Comunicado Técnico 128; Embrapa: Fortaleza, Brazil, 2007; 4p. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.S.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas pela Captura do Radical Livre DPPH; Comunicado Técnico 127; Embrapa: Fortaleza, Brazil, 2007; 4p. [Google Scholar]
- Garcia-Neto, M.; Perri, S.H.V. PPM: Practical Modeling Program. Available online: https://sites.google.com/site/programapraticodemodelagem/ (accessed on 12 January 2022).
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.I.S.; Nlemadim, B.C.; Davidson, D.L.W. Lipid peroxidation products and antioxidant proteins in plasma and cerebrospinal fluid from multiple sclerosis patients. Neurochem. Res. 1985, 10, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L., Jr. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- The Jamovi Project. Version 2.2.5 [Computer Software]. 2022. Available online: http://www.jamovi.org (accessed on 2 August 2022).
- Carmona-Jiménez, Y.; García-Moreno, M.V.; Igartuburu, J.M.; Garcia Barroso, C. Simplification of the DPPH assay for estimating the antioxidant activity of wine and wine by-products. Food Chem. 2014, 165, 198–204. [Google Scholar] [CrossRef]
- Roques, S.; Deborde, C.; Richard, N.; Skiba-Cassy, S.; Moing, A.; Fauconneau, B. Metabolomics and fish nutrition: A review in the context of sustainable feed development. Rev. Aquac. 2018, 12, 261–282. [Google Scholar] [CrossRef] [Green Version]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary Polyphenol Supplementation in Food Producing Animals: Effects on the Quality of Derived Products. Animals 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in Monogastric Nutrition—A Review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Neto, A.F.; Martins, C.; Schwegler, E.; Barcellos, J.; Barth, A. Blood metabolites and fecal starch as indicators of feed efficiency of beef cattle in the feedlot. Arq. Bras. Med. Vet. Zootec. 2021, 73, 1260–1268. [Google Scholar] [CrossRef]
- Davis, R.P.; Boyd, C.E.; Davis, D.A. Resource sharing and resource sparing, understanding the role of production intensity and farm practices in resource use in shrimp aquaculture. Ocean Coast. Manag. 2021, 207, 105595. [Google Scholar] [CrossRef]
- Sanches, E.G.; Silva, F.C.; Leite, J.R.; Silva, P.K.A.; Kerber, C.E.; Santos, P.A. A incorporação de óleo de peixe na dieta pode melhorar o desempenho da garoupa-verdadeira Epinephelus marginatus? Bol. Inst. Pesca 2014, 40, 147–155. [Google Scholar]
- Gomiero, L.M.; Braga, F.M.S. Relação peso-comprimento e fator de condição de Brycon opalinus (Pisces, Characiformes) no Parque Estadual da Serra do Mar-Núcleo Santa Virgínia, Mata Atlântica, Estado de São Paulo, Brasil. Acta Sci. Biol. Sci. 2006, 28, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Elkaradawy, A.; Abdel-Rahim, M.M.; Mohamed, R.A. Quillaja saponaria and/or linseed oil improved growth performance, water quality, welfare profile and immune-oxidative status of Nile tilapia, Oreochromis niloticus fingerlings. Aquac. Res. 2021, 53, 576–589. [Google Scholar] [CrossRef]
- Maduro, A.; da Silva, V.; Oliveira, R.; Barbosa, P. Perfil metabólico de filhotes de peixe-boi da Amazônia (Trichechus inunguis) em cativeiro, alimentados com diferentes sucedâneos do leite materno. Arq. Bras. Med. Vet. Zootec. 2020, 72, 1830–1838. [Google Scholar] [CrossRef]
- Melo, D.; Oliveira, D.; Melo, M.; Júnior, D.; Teixeira, E.; Guimarães, S. Perfil proteico de tilápia nilótica chitralada (Oreochromis niloticus), submetida ao estresse crônico por hipóxia. Arq. Bras. Med. Vet. Zootec. 2009, 61, 1183–1190. [Google Scholar] [CrossRef]
- Chernyavskikh, S.D.; Borodaeva, Z.A.; Borisovskiy, I.P.; Ostapenko, S.I.; Galtseva, O.A. Blood protein spectrum in representatives of the fish superclass. EurAsian J. BioSci. 2019, 13, 979–981. [Google Scholar]
- Rodrigues, G.M.; Nascimento, F.G.O.; Bizare, A.; Oliveira, W.J.; Guimarães, E.C.; Mundim, A.V. Perfil bioquímico sérico de tilápias do Nilo (Oreochromis niloticus) criadas em tanques rede durante as estações do verão e inverno. Acta Sci. Vet. 2018, 46, 1529. [Google Scholar] [CrossRef]
- Saha, P.; Talukdar, A.D.; Nath, R.; Sarker, S.D.; Nahar, L.; Sahu, J.; Choudhury, M.D. Role of Natural Phenolics in Hepatoprotection: A Mechanistic Review and Analysis of Regulatory Network of Associated Genes. Front. Pharmacol. 2019, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Montanha, F.P.; Pimpão, C.T. Toxicological effects of pyrethroids (Cypermethrin and Deltamethrin) in fish—Review. Rev. Electron. Vet. 2012, 9, 1–58. [Google Scholar]
- Honorato, C.A.; Ushizima, T.T.; Santamaria, F.M.; Flores-Quintana, C.I.; Marcondes, V.M.; Nascimento, C.A. Desempenho produtivo e econômica de surubins (Pseudoplatystoma sp.) alimentados com níveis de proteína e estocados em tanquerede. Arq. Bras. Med. Vet. Zootec. 2015, 67, 1408–1414. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, R.C.; Anami, J.M.; Steffens, C.A. Maçã: Compostos fenólicos e saúde. Rev. Interdisc. Est. Sal. UNIARP 2019, 9, 29–33. [Google Scholar] [CrossRef]
- Dehghani, F.; Morvaridzadeh, M.; Pizarro, A.B.; Rouzitalab, T.; Khorshidi, M.; Izadi, A.; Shidfar, F.; Omidi, A.; Heshmati, J. Effect of extra virgin olive oil consumption on glycemic control: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1953–1961. [Google Scholar] [CrossRef]
- García-Martínez, D.J.; Arroyo-Hernández, M.; Posada-Ayala, M.; Santos, C. The High Content of Quercetin and Catechin in Airen Grape Juice Supports Its Application in Functional Food Production. Foods 2021, 10, 1532. [Google Scholar] [CrossRef]
- Wagner, T.; Congleton, J.L. Blood chemistry correlates of nutritional condition, tissue damage, and stress in migrating juvenile chinook salmon (Oncorhynchus tshawytscha). Can. J. Fish. Aquat. Sci. 2004, 61, 1066–1074. [Google Scholar] [CrossRef]
- Coz-Rakovac, R.; Strunjak-Perovic, I.; Hacmanjek, M.; Popovic, N.T.; Lipej, Z.; Sostaric, B. Blood Chemistry and Histological Properties of Wild and Cultured Sea Bass (Dicentrarchus labrax) in the North Adriatic Sea. Vet. Res. Commun. 2005, 29, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Brum, A.; Pereira, S.A.; Cardoso, L.; Chagas, E.C.; Chaves, F.C.M.; Mouriño, J.L.P.; Martins, M.L. Blood biochemical parameters and melanomacrophage centers in Nile tilapia fed essential oils of clove basil and ginger. Fish Shellfish. Immunol. 2018, 74, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Wang, F.; Xing, C. A zebrafish (Danio rerio) model for high-throughput screening food and drugs with uric acid-lowering activity. Biochem. Biophys. Res. Commun. 2019, 508, 494–498. [Google Scholar] [CrossRef]
- Hong, J.; Chen, X.; Liu, S.; Fu, Z.; Han, M.; Wang, Y.; Gu, Z.; Ma, Z. Impact of fish density on water quality and physiological response of golden pompano (Trachinotus ovatus) flingerlings during transportation. Aquaculture 2019, 507, 260–265. [Google Scholar] [CrossRef]
- Hassanine, R.; Al-Hasawi, Z. Acanthocephalan Worms Mitigate the Harmful Impacts of Heavy Metal Pollution on Their Fish Hosts. Fishes 2021, 6, 49. [Google Scholar] [CrossRef]
- Randall, D.J.; Wright, P.A. Ammonia distribution and excretion in fish. Fish Physiol. Biochem. 1987, 3, 107–120. [Google Scholar] [CrossRef]
- Laganà, P.; Anastasi, G.; Marano, F.; Piccione, S.; Singla, R.K.; Dubey, A.K.; Delia, S.; Coniglio, M.A.; Facciolà, A.; Di Pietro, A.; et al. Phenolic Substances in Foods: Health Effects as Anti-Inflammatory and Antimicrobial Agents. J. AOAC Int. 2019, 102, 1378–1387. [Google Scholar] [CrossRef]
- Chen, C.Y.; Gregory, A.W.; Rodman, G.G.; Bowser, P.R.; Timmons, M.B. Blood chemistry of healthy, nephrocalcinosis-affected and ozone-treated tilapia in a recirculation system, with application of discriminant analysis. Aquaculture 2003, 218, 89–102. [Google Scholar] [CrossRef]
- Mauel, M.J.; Miller, D.L.; Merrill, A.L. Hematologic and plasma biochemical values of healthy hybrid tilapia (Oreochromis aureus × Oreochromis nilotica) maintained in a recirculating system. J. Zoo Wildl. Med. 2007, 38, 420–424. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Li, Z.-H.; Silovska, S.; Turek, J. Comparison of the effects of four anaesthetics on blood biochemical profiles and oxidative stress biomarkers in rainbow trout. Aquaculture 2011, 310, 369–375. [Google Scholar] [CrossRef]
- Cazenave, J.; de los Angeles Bistoni, A.; Pesce, S.F.; Wunderlin, D.A. Differential detoxification and antioxidant response in diverse organs of Corydoras paleatus experimentally exposed to microcystin-RR. Aquat. Toxicol. 2006, 76, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Zhang, X.; Xu, Y.; Liao, T.; Song, S.; Wang, J. Induction of hepatic enzymes and oxidative stress in Chinese rare minnow (Gobiocypris rarus) exposed to waterborne hexabromocyclododecane (HBCDD). Aquat. Toxicol. 2008, 86, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Kosmala, M.; Zduńczyk, Z.; Markowski, J. Grinding levels of raspberry pomace affect intestinal microbial activity, lipid and glucose metabolism in Wistar rats. Food Res. Int. 2019, 120, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Viji, P.; Binsi, P.K.; Visnuvinayagam, S.; Bindu, J.; Ravishankar, C.N.; Gopal, T.K.S. Efficacy of mint (Mentha arvensis) leaf and citrus (Citrus aurantium) peel extracts as natural preservatives for shelf life extension of chill stored Indian mackerel. J. Food Sci. Technol. 2015, 52, 6278–6289. [Google Scholar] [CrossRef] [PubMed]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Jiao, Y.; Kilmartin, P.A.; Fan, M.; Quek, S.Y. Assessment of phenolic contributors to antioxidant activity of new kiwifruit cultivars using cyclic voltammetry combined with HPLC. Food Chem. 2018, 268, 77–85. [Google Scholar] [CrossRef]
- da Silva, A.S.L.; Silva, A.D.J.; Latif, A.L.O.; Júnior, A.D.F.S.; Benevides, C.M.D.J. Uso de metodologias analíticas para determinação de compostos fenólicos em alimentos no Brasil: Avanços e fragilidades. Res. Soc. Dev. 2022, 11, e1311225193. [Google Scholar] [CrossRef]
- Koop, B.L.; da Silva, M.N.; da Silva, F.D.; Lima, K.T.d.S.; Soares, L.S.; de Andrade, C.J.; Valencia, G.A.; Monteiro, A.R. Flavonoids, anthocyanins, betalains, curcumin, and carotenoids: Sources, classification and enhanced stabilization by encapsulation and adsorption. Food Res. Int. 2022, 153, 110929. [Google Scholar] [CrossRef]
- Izar, M.C.d.O.; Lottenberg, A.M.; Giraldez, V.Z.R.; dos Santos, R.D.; Machado, R.M.; Bertolami, A.; Assad, M.H.V.; Saraiva, J.F.K.; Faludi, A.A.; Moreira, A.S.B.; et al. Posicionamento sobre o Consumo de Gorduras e Saúde Cardiovascular—2021. Arq. Bras. Cardiol. 2021, 116, 160–212. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Betts, J.A. Dietary sugars, exercise and hepatic carbohydrate metabolism. Proc. Nutr. Soc. 2019, 78, 246–256. [Google Scholar] [CrossRef]
- Luan, G.; Zhang, S.; Wang, M.; Lu, X. Progress and perspective on cyanobacterial glycogen metabolism engineering. Biotechnol. Adv. 2019, 37, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.J.S.; Façanha, N.P.B.; da Silva, E.M.H.; Carmo, E.T.S.D.; Barros, C.d.S.; da Silva, G.A. Yield and Characterization of the Centesimal Composition of Amazonian Estuarine Fish. J. Agric. Sci. Technol. B 2019, 9, 175–183. [Google Scholar] [CrossRef]
- Spitz, J.; Mourocq, E.; Schoen, V.; Ridoux, V. Proximate composition and energy content of forage species from the Bay of Biscay: High- or low-quality food? ICES J. Mar. Sci. 2010, 67, 909–915. [Google Scholar] [CrossRef] [Green Version]
- Viana, Z.C.V.; Silva, E.; Fernandes, G.B.; Santos, V.L.C.S. Centesimal composition in fish muscle in the Coast of the State of Bahia/Brazil. J. Med. Biol. Sci. 2013, 12, 157–162. [Google Scholar] [CrossRef]
- Li, T.; Kuang, S.; Xiao, T.; Hu, L.; Nie, P.; Ramaswamy, H.S.; Yu, Y. The Effect of Pressure–Shift Freezing versus Air Freezing and Liquid Immersion on the Quality of Frozen Fish during Storage. Foods 2022, 11, 1842. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic Review of Phenolic Compounds in Apple Fruits: Compositions, Distribution, Absorption, Metabolism, and Processing Stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef]

| Ingredients | ACM | APM | GRM | C |
|---|---|---|---|---|
| Corn meal | 39.00% | 39.00% | 39.00% | 44.10% |
| Soybean meal | 42.00% | 42.00% | 42.00% | 40.00% |
| Meat and bone meal | 5.58% | 5.58% | 5.58% | 6.99% |
| Viscera meal | 3.00% | 3.00% | 3.00% | 4.00% |
| Brown rice meal | 0.00% | 0.00% | 0.00% | 0.02% |
| Soybean oil | 3.60% | 3.60% | 3.60% | 3.00% |
| Lysine | 0.50% | 0.50% | 0.50% | 0.50% |
| Methionine | 0.10% | 0.10% | 0.10% | 0.10% |
| Threonine | 0.10% | 0.10% | 0.10% | 0.10% |
| Tryptophan | 0.10% | 0.10% | 0.10% | 0.01% |
| Mineral and vitamin premix * | 1.00% | 1.00% | 1.00% | 1.00% |
| NaCl | 0.00% | 0.00% | 0.00% | 0.16% |
| Vitamin C | 0.02% | 0.02% | 0.02% | 0.02% |
| Acerola meal | 5.00% | 0.00% | 0.00% | 0.00% |
| Apple meal | 0.00% | 5.00% | 0.00% | 0.00% |
| Grape meal | 0.00% | 0.00% | 5.00% | 0.00% |
| Nutrients/energy—Calculated values | ||||
| Digestible energy | 3100.88 | 3100.57 | 3100.01 | 3100.65 |
| Crude protein (%) | 27.309 | 27.109 | 27.167 | 27.379 |
| Digestible energy (%) | 24.518 | 24.769 | 24.728 | 25.069 |
| Arginine (%) | 21.588 | 21.588 | 21.588 | 2.28 |
| Lysine (%) | 22.015 | 22.015 | 22.015 | 2.27 |
| Methionine (%) | 0.4908 | 0.4908 | 0.4908 | 0.52 |
| Threonine (%) | 12.112 | 12.112 | 12.112 | 1.28 |
| Tryptophan (%) | 0.4957 | 0.4957 | 0.4957 | 0.50 |
| Mineral matter (%) | 58.689 | 58.689 | 58.689 | 6.63 |
| Calcium (%) | 10.472 | 10.472 | 10.472 | 1.26 |
| Phosphorus (%) | 0.8365 | 0.8365 | 0.8365 | 0.94 |
| Vitamin C (mg/kg) | 608.4 | 608.4 | 608.4 | 608.4 |
| Bioactive Compounds | Meals | p-Value | ||
|---|---|---|---|---|
| ACM | APM | GRM | ||
| TPC (μg/100 mg) | 772 ± 7.2 b | 261 ± 78.4 c | 857 ± 2.6 a | <0.001 |
| Carotenoids (mg/kg) | 2.87 ± 0.55 b | 4.03 ± 0.56 ab | 5.76 ± 0.58 a | 0.006 |
| Antioxidant capacity | ||||
| ABTS (μM Trolox/g) | 20.7 ± 1.55 b | 3.4 ± 1.31 c | 132.9 ± 4.98 a | <0.001 |
| DPPH (EC50 mg/L) | 670 ± 4.03 b | 733 ± 5.12 a | 463 ± 4.03 b | <0.001 |
| Parameters | Diets | p Value | |||
|---|---|---|---|---|---|
| ACM | APM | GRM | C | ||
| Weight gain (g) | 591.00 ± 38 a | 491.00 ± 21.9 bc | 471.00 ± 32.8 c | 531.00 ± 48.8 b | 0.002 |
| Feed efficiency | 0.095 ± 0.028 | 0.061 ± 0.039 | 0.107 ± 0.046 | 0.095 ± 0.055 | 0.310 |
| Body condition factor | 2.706 ± 0.155 | 2.666 ± 0.291 | 2.768 ± 0.172 | 2.370 ± 0.616 | 0.198 |
| Hepatosomatic index | 1.156 ± 0.344 | 1.181 ± 0.369 | 1.275 ± 0.424 | 1.179 ± 0.403 | 0.880 |
| Parameters | Diets | Reference Limits | p Value | |||
|---|---|---|---|---|---|---|
| ACM | APM | GRM | C | |||
| Total protein (g/dL) | 3.59 ± 0.53 | 3.31 ± 1.16 | 3.64 ± 0.62 | 3.71 ± 0.71 | 3.3–5.0 g/dL | 0.538 |
| Albumin (g/dL) | 1.31 ± 0.31 | 1.16 ± 0.47 | 1.31 ± 0.33 | 1.46 ± 0.39 | 1.1–1.7 g/dL | 0.166 |
| Globulin (g/dL) | 2.27 ± 0.32 b | 1.82 ± 0.54 a | 2.24 ± 0.30 b | 2.25 ± 0.41 b | 1.3–3.1 g/dL | 0.012 |
| Albumin/globulin | 0.58 ± 0.13 | 0.61 ± 0.18 | 0.55 ± 0.10 | 0.66 ± 0.13 | 0.4–0.8 | 0.084 |
| Alanine aminotransferase (ALT) (U/L) | 81.2 ± 22.4 | 81.9 ± 13.8 | 75.2 ± 12.9 | 84.1 ± 19.2 | 28.3–121 U/L | 0.253 |
| Aspartate aminotransferase (AST) (U/L) | 74.7 ± 25.3 | 75.7 ± 19.4 | 67.0 ± 12.9 | 81.7 ± 22.0 | 16–120 U/L | 0.052 |
| Alkaline phosphatase (U/L) | 41.9 ± 14.3 a | 53.1 ± 19.1 ab | 39.5 ± 11.9 a | 59.2 ± 28.0 b | 16–38 U/L | 0.005 |
| Glucose (mg/dL) | 59.6 ± 19.0 a | 68.0 ± 17.7 ab | 87.3 ± 35.0 b | 65.1 ± 25.4 a | 52–156 mg/dL | <0.001 |
| Cholesterol (mg/dL) | 138 ± 23.9 | 137 ± 45.9 | 155 ± 40.0 | 152 ± 31.1 | 88–228 mg/dL | 0.206 |
| Triglycerides (mg/dL) | 143 ± 34.0 | 123 ± 81.9 | 131 ± 61.7 | 158 ± 48.7 | * | 0.376 |
| Uric acid (mg/dL) | 2.30 ± 1.04 a | 3.23 ± 1.49 ab | 2.25 ± 0.88 a | 4.04 ± 1.85 b | * | <0.001 |
| Creatinine (mg/dL) | 0.154 ± 0.05 a | 0.221 ± 0.11 b | 0.181 ± 0.06 ab | 0.179 ± 0.04 ab | 0–0.8 mg/dL | 0.031 |
| Urea (mg/dL) | 2.73 ± 0.58 b | 2.29 ± 0.88 ab | 3.55 ± 1.09 c | 1.85 ± 0.52 a | 1.0–4.0 mg/dL | <0.001 |
| TBARS (ng MDA/mL) | 3.96 ± 2.31 a | 5.14 ± 3.86 a | 3.14 ± 1.83 a | 8.51 ± 5.12 b | * | <0.001 |
| TAS (mmol/L) | 0.576 ± 0.10 a | 0.558 ± 0.06 a | 0.594 ± 0.06 a | 0.464 ± 0.04 b | * | <0.001 |
| TOS (mmol/L) | 0.05 ± 0.006 a | 0.05 ± 0.002 a | 0.05 ± 0.004 a | 0.06 ± 0.003 b | * | <0.001 |
| Parameters | Diets | p Value | |||
|---|---|---|---|---|---|
| ACM | APM | GRM | C | ||
| Moisture (%) | 78.18 ± 0.93 bc | 79.12 ± 1.21 a | 78.78 ± 0.80 ab | 77.90 ± 0.94 c | <0.001 |
| Proteins (%) | 15.67 ± 0.99 ab | 14.85 ± 0.63 b | 16.55 ± 0.31 a | 15.01 ± 1.4 b | <0.001 |
| Carbohydrates (%) | 1.76 ± 0,82 b | 1.73 ± 0.80 b | 0.57 ± 0.28 c | 3.66 ± 1.24 a | <0.001 |
| Lipids (%) | 3.17 ± 0.86 a | 3.14 ± 0.56 a | 2.83 ± 0.12 a | 2.21 ± 0.38 b | <0.001 |
| Ashes (%) | 1.23 ± 0.74 ab | 1.17 ± 0.9 b | 1.26 ± 0.06 a | 1.28 ± 0.14 a | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotolli, A.P.; da Fonseca, V.E.; Bermejo-Poza, R.; Ferraz, I.G.; de Souza, L.C.C.; Brasil, M.L.; Santana, R.F.; Games, I.M.M.; Ferraz, M.C.; Theophilo, G.; et al. Dietary Fruit By-Products Improve the Physiological Status of Nile Tilapias (Oreochromis niloticus) and the Quality of Their Meat. Antioxidants 2023, 12, 1607. https://doi.org/10.3390/antiox12081607
Chotolli AP, da Fonseca VE, Bermejo-Poza R, Ferraz IG, de Souza LCC, Brasil ML, Santana RF, Games IMM, Ferraz MC, Theophilo G, et al. Dietary Fruit By-Products Improve the Physiological Status of Nile Tilapias (Oreochromis niloticus) and the Quality of Their Meat. Antioxidants. 2023; 12(8):1607. https://doi.org/10.3390/antiox12081607
Chicago/Turabian StyleChotolli, Andrey P., Victor E. da Fonseca, Rubén Bermejo-Poza, Isabella G. Ferraz, Letícia C. C. de Souza, Mariana L. Brasil, Ronnie F. Santana, Isadora M. M. Games, Murilo C. Ferraz, Gabrielly Theophilo, and et al. 2023. "Dietary Fruit By-Products Improve the Physiological Status of Nile Tilapias (Oreochromis niloticus) and the Quality of Their Meat" Antioxidants 12, no. 8: 1607. https://doi.org/10.3390/antiox12081607
APA StyleChotolli, A. P., da Fonseca, V. E., Bermejo-Poza, R., Ferraz, I. G., de Souza, L. C. C., Brasil, M. L., Santana, R. F., Games, I. M. M., Ferraz, M. C., Theophilo, G., Salmaso, P. H. L., Balbino, A. L. S., dos Santos, F. D. R., & Ponsano, E. H. G. (2023). Dietary Fruit By-Products Improve the Physiological Status of Nile Tilapias (Oreochromis niloticus) and the Quality of Their Meat. Antioxidants, 12(8), 1607. https://doi.org/10.3390/antiox12081607