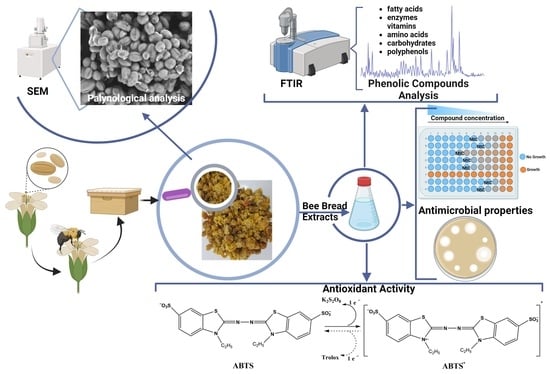
Bee Bread: A Promising Source of Bioactive Compounds with Antioxidant Properties—First Report on Some Antimicrobial Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reagents
2.3. Palynological Analysis
2.4. Bee Bread Extract (BBE) Preparation
2.5. Chemical Characterization of BBE
2.5.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.2. Determination of Total Phenols Content (TPC)
2.5.3. Determination of Total Flavonoids Content (TFC)
2.5.4. Phenolic Compound Analysis by UHPLC-MS/MS
2.5.5. Determination of Trolox Equivalent Antioxidant Capacity (TEAC)
2.6. Methods Applied in the Biological Activity of BBE
2.6.1. Qualitative Assessment of the Antimicrobial Activity
2.6.2. Quantitative Assessment of the Antimicrobial Activity
2.6.3. Semiquantitative Assessment of the Microbial Adherence to the Inert Substratum
2.7. Statistical Analysis
3. Results and Discussions
3.1. Palynological Analysis

3.2. Chemical Composition of BBE
3.2.1. FTIR Spectroscopy
3.2.2. Determinations of TPC, TFC, and TEAC
3.2.3. Phenolic Compound Profiles by UHPLC-DAD-ESI/MS
3.3. Biological Activity of BBE
3.3.1. Qualitative Assessment of the Antimicrobial Activity
3.3.2. Quantitative Evaluation of Antimicrobial Activity
3.3.3. Semiquantitative Assay of the Microbial Adherence to the Inert Substratum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nayik, G.A.; Shah, T.R.; Muzaffar, K.; Wani, S.A.; Gull, A.; Majid, I.; Bhat, F.M. Honey: Its History And Religious Significance: A Review. Univers. J. Pharm. 2014, 3, 5–8. [Google Scholar]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee Pollen: Current Status and Therapeutic Potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef]
- Madras-Majewska, B.; Majewski, J. Production and Prices of Honey in Researched Apriaries in Poland. Sci. Yearb. Assoc. Agric. Agribus. Econ. 2007, 9, 298–302. [Google Scholar]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Honey, Wound Repair and Regenerative Medicine. J. Funct. Biomater. 2018, 9, 34. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elashal, M.; Kieliszek, M.; Ghazala, N.E.; Farag, M.A.; Saeed, A.; Xiao, J.; Zou, X.; Khatib, A.; Göransson, U.; et al. Recent insights into chemical and pharmacological studies of bee bread. Trends Food Sci. Technol. 2020, 97, 300–316. [Google Scholar] [CrossRef]
- Margaoan, R.; Strant, M.; Varadi, A.; Topal, E.; Yucel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef]
- Ilie, C.I.; Oprea, E.; Geana, E.I.; Spoiala, A.; Buleandra, M.; Gradisteanu Pircalabioru, G.; Badea, I.A.; Ficai, D.; Andronescu, E.; Ficai, A.; et al. Bee Pollen Extracts: Chemical Composition, Antioxidant Properties, and Effect on the Growth of Selected Probiotic and Pathogenic Bacteria. Antioxidants 2022, 11, 959. [Google Scholar] [CrossRef]
- Spoiala, A.; Ilie, C.I.; Ficai, D.; Ficai, A.; Andronescu, E. Synergic Effect of Honey with Other Natural Agents in Developing Efficient Wound Dressings. Antioxidants 2022, 12, 34. [Google Scholar] [CrossRef]
- Ioniță-Mîndrican, C.-B.; Mititelu, M.; Musuc, A.M.; Oprea, E.; Ziani, K.; Neacșu, S.M.; Grigore, N.D.; Negrei, C.; Dumitrescu, D.-E.; Mireșan, H.; et al. Honey and Other Beekeeping Products Intake among the Romanian Population and Their Therapeutic Use. Appl. Sci. 2022, 12, 9649. [Google Scholar] [CrossRef]
- Bakour, M.; Al-Waili, N.S.; El Menyiy, N.; Imtara, H.; Figuira, A.C.; Al-Waili, T.; Lyoussi, B. Antioxidant activity and protective effect of bee bread (honey and pollen) in aluminum-induced anemia, elevation of inflammatory makers and hepato-renal toxicity. J. Food Sci. Technol. 2017, 54, 4205–4212. [Google Scholar] [CrossRef] [PubMed]
- Aylanc, V.; Falcao, S.I.; Vilas-Boas, M. Bee pollen and bee bread nutritional potential: Chemical composition and macronutrient digestibility under in vitro gastrointestinal system. Food Chem. 2023, 413, 135597. [Google Scholar] [CrossRef] [PubMed]
- Ćirić, J.; Haneklaus, N.; Rajić, S.; Baltić, T.; Lazić, I.B.; Đorđević, V. Chemical composition of bee bread (perga), a functional food: A review. J. Trace Elem. Miner. 2022, 2, 100038. [Google Scholar] [CrossRef]
- Bakour, M.; Fernandes, Â.; Barros, L.; Sokovic, M.; Ferreira, I.C.F.R.; Badiaa, l. Bee bread as a functional product: Chemical composition and bioactive properties. LWT 2019, 109, 276–282. [Google Scholar] [CrossRef]
- Urcan, A.C.; Marghitas, L.A.; Dezmirean, D.S.; Bobis, O.; Bonta, V.; Muresan, C.I.; Margaoan, R. Chemical Composition and Biological Activities of Beebread—Review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2017, 74, 6. [Google Scholar] [CrossRef]
- Urcan, A.C.; Criste, A.D.; Dezmirean, D.S.; Bobiș, O.; Bonta, V.; Dulf, F.V.; Mărgăoan, R.; Cornea-Cipcigan, M.; Campos, M.G. Botanical origin approach for a better understanding of chemical and nutritional composition of beebread as an important value-added food supplement. LWT 2021, 142, 111068. [Google Scholar] [CrossRef]
- Mayda, N.; Özkök, A.; Ecem Bayram, N.; Gerçek, Y.C.; Sorkun, K. Bee bread and bee pollen of different plant sources: Determination of phenolic content, antioxidant activity, fatty acid and element profiles. J. Food Meas. Charact. 2020, 14, 1795–1809. [Google Scholar] [CrossRef]
- Adaskeviciute, V.; Kaskoniene, V.; Kaskonas, P.; Barcauskaite, K.; Maruska, A. Comparison of Physicochemical Properties of Bee Pollen with Other Bee Products. Biomolecules 2019, 9, 819. [Google Scholar] [CrossRef]
- Kutlu, N.; Gerçek, Y.C.; Celik, S.; Bayram, S.; Bayram, N.E. An optimization study for amino acid extraction from bee bread using choline chloride-acetic acid deep eutectic solvent and determination of individual phenolic profile. J. Food Meas. Charact. 2023, 18, 1026–1037. [Google Scholar] [CrossRef]
- Tomás, A.; Falcão, S.I.; Russo-Almeida, P.; Vilas-Boas, M. Potentialities of beebread as a food supplement and source of nutraceuticals: Botanical origin, nutritional composition and antioxidant activity. J. Apic. Res. 2017, 56, 219–230. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Aylanc, V.; Falcão, S.I.; Ertosun, S.; Vilas-Boas, M. From the hive to the table: Nutrition value, digestibility and bioavailability of the dietary phytochemicals present in the bee pollen and bee bread. Trends Food Sci. Technol. 2021, 109, 464–481. [Google Scholar] [CrossRef]
- Othman, Z.A.; Wan Ghazali, W.S.; Noordin, L.; Mohd Yusof, N.A.; Mohamed, M. Phenolic Compounds and the Anti-Atherogenic Effect of Bee Bread in High-Fat Diet-Induced Obese Rats. Antioxidants 2019, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin E in Human Health and Some Diseases. SQU Med. J. 2014, 14, 157–163. [Google Scholar]
- Didaras, N.A.; Kafantaris, I.; Dimitriou, T.G.; Mitsagga, C.; Karatasou, K.; Giavasis, I.; Stagos, D.; Amoutzias, G.D.; Hatjina, F.; Mossialos, D. Biological Properties of Bee Bread Collected from Apiaries Located across Greece. Antibiotics 2021, 10, 555. [Google Scholar] [CrossRef] [PubMed]
- Ben Bacha, A.; Norah, A.O.; Al-Osaimi, M.; Harrath, A.H.; Mansour, L.; El-Ansary, A. The therapeutic and protective effects of bee pollen against prenatal methylmercury induced neurotoxicity in rat pups. Metab. Brain Dis. 2020, 35, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Eleazu, C.; Suleiman, J.B.; Othman, Z.A.; Zakaria, Z.; Nna, V.U.; Hussain, N.H.N.; Mohamed, M. Bee bread attenuates high fat diet induced renal pathology in obese rats via modulation of oxidative stress, downregulation of NF-kB mediated inflammation and Bax signalling. Arch. Physiol. Biochem. 2022, 128, 1088–1104. [Google Scholar] [CrossRef] [PubMed]
- Kosedag, M.; Gulaboglu, M. Pollen and bee bread expressed highest anti-inflammatory activities among bee products in chronic inflammation: An experimental study with cotton pellet granuloma in rats. Inflammopharmacology 2023, 31, 1967–1975. [Google Scholar] [CrossRef]
- Hsu, C.K.; Wang, D.Y.; Wu, M.C. A Potential Fungal Probiotic Aureobasidium melanogenum CK-CsC for the Western Honey Bee, Apis mellifera. J. Fungi 2021, 7, 508. [Google Scholar] [CrossRef]
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives. Antibiotics 2020, 9, 811. [Google Scholar] [CrossRef]
- Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Ghouizi, A.; Es-Safi, I.; Mechchate, H.; Lyoussi, B. Bee Bread as a Promising Source of Bioactive Molecules and Functional Properties: An Up-To-Date Review. Antibiotics 2022, 11, 203. [Google Scholar] [CrossRef]
- Barta, D.G.; Cornea-Cipcigan, M.; Margaoan, R.; Vodnar, D.C. Biotechnological Processes Simulating the Natural Fermentation Process of Bee Bread and Therapeutic Properties-An Overview. Front. Nutr. 2022, 9, 871896. [Google Scholar] [CrossRef]
- Toutiaee, S.; Mojgani, N.; Harzandi, N.; Moharrami, M.; Mokhberosafa, L. In vitro probiotic and safety attributes of Bacillus spp. isolated from beebread, honey samples and digestive tract of honeybees Apis mellifera. Lett. Appl. Microbiol. 2022, 74, 656–665. [Google Scholar] [CrossRef]
- Bleha, R.; Shevtsova, T.; Kruzík, V.; Skorpilová, T.; Salon, I.; Erban, V.; Brindza, J.; Brovarskyi, V.; Sinica, A. Bee breads from Eastern Ukraine: Composition, physical properties and biological activities. Czech J. Food Sci. 2019, 37, 9–20. [Google Scholar] [CrossRef]
- Schaad, E.; Fracheboud, M.; Droz, B.; Kast, C. Quantitation of pesticides in bee bread collected from honey bee colonies in an agricultural environment in Switzerland. Environ. Sci. Pollut. R. 2023, 30, 56353–56367. [Google Scholar] [CrossRef]
- Murcia-Morales, M.; Heinzen, H.; Parrilla-Vázquez, P.; Gómez-Ramos, M.d.M.; Fernández-Alba, A.R. Presence and distribution of pesticides in apicultural products: A critical appraisal. TrAC Trends Anal. Chem. 2022, 146, 116506. [Google Scholar] [CrossRef]
- Zuluaga, C.M.; Serrato, J.C.; Quicazan, M.C. Chemical, Nutritional and Bioactive Characterization of Colombian Bee-Bread. Chem. Eng. Trans. 2015, 43, 175–180. [Google Scholar] [CrossRef]
- Ivanišová, E.; Kačániová, M.; Frančáková, H.; Petrová, J.; Hutková, J.; Brovarskyi, V.; Velychko, S.; Adamchuk, L.; Schubertová, Z.; Musilová, J. Bee bread—Perspective source of bioactive compounds for future. Potravin. Slovak. J. Food Sci. 2015, 9, 592–598. [Google Scholar] [CrossRef]
- Baltrusaityte, V.; Venskutonis, P.R.; Ceksteryte, V. Antibacterial Activity of Honey and Beebread of Different Origin against S. aureus and S. epidermidis. Food Technol. Biotechnol. 2007, 45, 201–208. [Google Scholar]
- Sobral, F.; Calhelha, R.C.; Barros, L.; Duenas, M.; Tomas, A.; Santos-Buelga, C.; Vilas-Boas, M.; Ferreira, I.C. Flavonoid Composition and Antitumor Activity of Bee Bread Collected in Northeast Portugal. Molecules 2017, 22, 248. [Google Scholar] [CrossRef]
- Bayram, N.E.; Gercek, Y.C.; Çelik, S.; Mayda, N.; Kostić, A.Ž.; Dramićanin, A.M.; Özkök, A. Phenolic and free amino acid profiles of bee bread and bee pollen with the same botanical origin—Similarities and differences. Arab. J. Chem. 2021, 14, 103004–103016. [Google Scholar] [CrossRef]
- Morais, M.; Moreira, L.; Feas, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef]
- Pollen Atlas. 2023. Available online: https://pollenatlas.net/ (accessed on 1 February 2023).
- PalDat—A Palynological Database. Available online: www.paldat.org (accessed on 1 February 2023).
- Halbritter, H.; Grímsson, S.U.F.; Weber, M.; Hesse, R.Z.M.; Buchner, R.; Frosch-Radivo, M.S.A. Illustrated Pollen Terminology, 2nd ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Klimko, M.; Nowińska, R.; Wilkin, P.; Wiland-Szymańska, J. Pollen Morphology of Some Species of the Genus Sansevieria Petagna (Asparagaceae). Acta Biol. Cracoviensia S. Bot. 2017, 59, 63–75. [Google Scholar] [CrossRef]
- Rahmawati, L.U.; Purwanti, E.; Budiyanto, M.A.K.; Zaenab, S.; Susetyarini, R.E.; Permana, T.I. Identification of Pollen Grains Morphology and Morphometry in Liliaceae. Int. Conf. Life Sci. Technol. Ser. Earth Environ. Sci. 2019, 276, 012031. [Google Scholar] [CrossRef]
- Peng, Y.; Pu, X.; Yu, Q.; Zhou, H.; Huang, T.; Xu, B.; Gao, X. Comparative Pollen Morphology of Selected Species of Blumea DC. and Cyathocline Cass. and Its Taxonomic Significance. Plants 2023, 12, 2909. [Google Scholar] [CrossRef]
- Spoiala, A.; Ilie, C.I.; Dolete, G.; Petrisor, G.; Trusca, R.D.; Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Ditu, M.L. The Development of Alginate/Ag NPs/Caffeic Acid Composite Membranes as Adsorbents for Water Purification. Membranes 2023, 13, 591. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Marinas, I.; Oprea, E.; Geana, E.-I.; Chifiriuc, C.; Lazar, V. Antimicrobial and antioxidant activity of the vegetative and reproductive organs of Robinia pseudoacacia. J. Serb. Chem. Soc. 2014, 79, 1363–1378. [Google Scholar] [CrossRef]
- Alizadeh, A.; Alizadeh, O.; Amari, G.; Zare, M. Essential Oil Composition, Total Phenolic Content, Antioxidant Activity and Antifungal Properties of Iranian Thymus daenensi ssubsp. daenensis Celak. as in Influenced by Ontogenetical Variation. J. Essent. Oil Bear. Plants 2013, 16, 59–70. [Google Scholar] [CrossRef]
- Ciucure, C.T.; Geana, E.I. Phenolic compounds profile and biochemical properties of honeys in relationship to the honey floral sources. Phytochem. Anal. 2019, 30, 481–492. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Rio, D.D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Trusca, R.D.; Ilie, C.I.; Oprea, O.C.; Andronescu, E. Innovative Antimicrobial Chitosan/ZnO/Ag NPs/Citronella Essential Oil Nanocomposite-Potential Coating for Grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef]
- Spoiala, A.; Ilie, C.I.; Trusca, R.D.; Oprea, O.C.; Surdu, V.A.; Vasile, B.S.; Ficai, A.; Ficai, D.; Andronescu, E.; Ditu, L.M. Zinc Oxide Nanoparticles for Water Purification. Materials 2021, 14, 4747–4763. [Google Scholar] [CrossRef]
- Performance Standards for Antimicrobial Susceptibility Testing. In CLSI Supplemenent M100; Clinical and Laboratory Standards Institute: San Antonio, TX, USA, 2021; Volume 41, p. 352.
- Halbritter, H.; Grímsson, S.U.F.; Weber, M.; Hesse, R.Z.M.; Buchner, R.; Frosch-Radivo, M.S.A. Pollen Morphology and Ultrastructure. In Illustrated Pollen Terminology, 2nd ed.; Springer: Cham, Switzerland, 2018; pp. 37–67. [Google Scholar] [CrossRef]
- Oroian, M.; Dranca, F.; Ursachi, F. Characterization of Romanian Bee Pollen-An Important Nutritional Source. Foods 2022, 11, 2633. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Özkök, A.; Keskin, Ş.; Mayda, N.; Urcan, A.C.; Cornea-Cipcigan, M. Bee collected pollen as a value-added product rich in bioactive compounds and unsaturated fatty acids: A comparative study from Turkey and Romania. Food Sci. Technol. 2021, 149, 111925–111936. [Google Scholar] [CrossRef]
- Spulber, R.; Dogaroglu, M.; Babeanu, N.; Popa, O. Physicochemical characteristics of fresh bee pollen from different botanical origins. Rom. Biotechnol. Lett. 2018, 23, 13357–13365. [Google Scholar]
- Isopescu, R.D.; Spulber, R.; Josceanu, A.M.; Mihaiescu, D.E.; Popa, O. Romanian bee pollen classification and property modelling. J. Apic. Res. 2020, 59, 443–451. [Google Scholar] [CrossRef]
- Li, J.-L.; Li, W.-L.; Zhang, J.; Pang, Y.-T.; Xiong, J.; Wu, P.; Wei, B.-R.; Li, X.-J.; Huang, Q.; Tang, Q.-H.; et al. Seasonal Dynamics of the Microbiota and Nutritional Composition in Bee Bread from Apis cerana and Apis mellifera Colonies. Food Res. Int. 2023, 113905. [Google Scholar] [CrossRef]
- Leponiemi, M.; Freitak, D.; Moreno-Torres, M.; Pferschy-Wenzig, E.M.; Becker-Scarpitta, A.; Tiusanen, M.; Vesterinen, E.J.; Wirta, H. Honeybees’ foraging choices for nectar and pollen revealed by DNA metabarcoding. Sci. Rep. 2023, 13, 14753. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Ursachi, F.; Dranca, F. Ultrasound-Assisted Extraction of Polyphenols from Crude Pollen. Antioxidants 2020, 9, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Astolfi, P.; Conti, C.; Monaci, E.; Stefano, M.; Carloni, P. Morphological, Physicochemical and FTIR Spectroscopic Properties of Bee Pollen Loads from Different Botanical Origin. Molecules 2019, 24, 3974. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Ursachi, F.; Oroian, M. Bee Bread: Physicochemical Characterization and Phenolic Content Extraction Optimization. Foods 2020, 9, 1358. [Google Scholar] [CrossRef]
- Kasprzyk, I.; Depciuch, J.; Grabek-Lejko, D.; Parlinska-Wojtan, M. FTIR-ATR spectroscopy of pollen and honey as a tool for unifloral honey authentication. The case study of rape honey. Food Control 2018, 84, 33–40. [Google Scholar] [CrossRef]
- Pini, R.; Furlanetto, G.; Castellano, L.; Saliu, F.; Rizzi, A.; Tramelli, A. Effects of stepped-combustion on fresh pollen grains: Morphoscopic, thermogravimetric, and chemical proxies for the interpretation of archeological charred assemblages. Rev. Palaeobot. Palynol. 2018, 259, 142–158. [Google Scholar] [CrossRef]
- Treml, J.; Lelakova, V.; Smejkal, K.; Paulickova, T.; Labuda, S.; Granica, S.; Havlik, J.; Jankovska, D.; Padrtova, T.; Hosek, J. Antioxidant Activity of Selected Stilbenoid Derivatives in a Cellular Model System. Biomolecules 2019, 9, 468. [Google Scholar] [CrossRef]
- Julia, M.; Eugenia Marta, K.; María José, N.; Agustín, G.A. Antioxidant Capacity of Anthocyanin Pigments. In Flavonoids; Goncalo, C.J., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 11. [Google Scholar]
- Srivastava, R. Physicochemical, antioxidant properties of carotenoids and its optoelectronic and interaction studies with chlorophyll pigments. Sci. Rep. 2021, 11, 18365. [Google Scholar] [CrossRef]
- Bechir, B.; Imen, R. Potential Antioxidant Activity of Terpenes. In Terpenes and Terpenoids; Shagufta, P., Areej Mohammad, A.-T., Eds.; IntechOpen: Rijeka, Croatia, 2021; Chapter 5. [Google Scholar]
- Rocchetti, G.; Senizza, B.; Zengin, G.; Okur, M.A.; Montesano, D.; Yildiztugay, E.; Lobine, D.; Mahomoodally, M.F.; Lucini, L. Chemical Profiling and Biological Properties of Extracts from Different Parts of Colchicum szovitsii Subsp. Szovitsii. Antioxidants 2019, 8, 632. [Google Scholar] [CrossRef]
- Tawfik, A.I.; Ahmed, Z.H.; Abdel-Rahman, M.F.; Moustafa, A.M. Effect of some bee bread quality on protein content and antioxidant system of honeybee workers. Int. J. Trop. Insect Sci. 2022, 43, 93–105. [Google Scholar] [CrossRef]
- Gercek, Y.C.; Celik, S.; Bayram, S. Screening of Plant Pollen Sources, Polyphenolic Compounds, Fatty Acids and Antioxidant/Antimicrobial Activity from Bee Pollen. Molecules 2021, 27, 117. [Google Scholar] [CrossRef]
- Jara, C.; Leyton, M.; Osorio, M.; Silva, V.; Fleming, F.; Paz, M.; Madrid, A.; Mellado, M. Antioxidant, phenolic and antifungal profiles of Acanthus mollis (Acanthaceae). Nat. Prod. Res. 2017, 31, 2325–2328. [Google Scholar] [CrossRef]
- Castiglioni, S.; Stefano, M.; Astolfi, P.; Pisani, M.; Carloni, P. Characterisation of Bee Pollen from the Marche Region (Italy) According to the Botanical and Geographical Origin with Analysis of Antioxidant Activity and Colour, Using a Chemometric Approach. Molecules 2022, 27, 7996. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gambacorta, G.; Tlais, A.Z.A.; Cantatore, V.; Gobbetti, M. Volatilome and Bioaccessible Phenolics Profiles in Lab-Scale Fermented Bee Pollen. Foods 2021, 10, 286. [Google Scholar] [CrossRef]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernandez, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Wojtczuk, M.; Roszko, M.; Bryla, M.; Trajkovska Petkoska, A. Recent advances and opportunities related to the use of bee products in food processing. Food Sci. Nutr. 2023, 11, 4372–4397. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Q.; Liu, R. Widely targeted metabolomics analysis reveals the effect of fermentation on the chemical composition of bee pollen. Food Chem. 2022, 375, 131908. [Google Scholar] [CrossRef]
- Santos, A.C.D.; Biluca, F.C.; Braghini, F.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Phenolic composition and biological activities of stingless bee honey: An overview based on its aglycone and glycoside compounds. Food Res. Int. 2021, 147, 110553. [Google Scholar] [CrossRef]
- Negri, P.; Maggi, M.D.; Ramirez, L.; De Feudis, L.; Szwarski, N.; Quintana, S.; Eguaras, M.J.; Lamattina, L. Abscisic acid enhances the immune response in Apis mellifera and contributes to the colony fitness. Apidologie 2015, 46, 542–557. [Google Scholar] [CrossRef]
- Ramirez, L.; Negri, P.; Sturla, L.; Guida, L.; Vigliarolo, T.; Maggi, M.; Eguaras, M.; Zocchi, E.; Lamattina, L. Abscisic acid enhances cold tolerance in honeybee larvae. Proc. Biol. Sci. 2017, 284, 20162140. [Google Scholar] [CrossRef]
- Szawarski, N.; Saez, A.; Dominguez, E.; Dickson, R.; De Matteis, A.; Eciolaza, C.; Justel, M.; Aliano, A.; Solar, P.; Bergara, I.; et al. Effect of Abscisic Acid (ABA) Combined with Two Different Beekeeping Nutritional Strategies to Confront Overwintering: Studies on Honey Bees’ Population Dynamics and Nosemosis. Insects 2019, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Balino, P.; Gomez-Cadenas, A.; Lopez-Malo, D.; Romero, F.J.; Muriach, M. Is There A Role for Abscisic Acid, A Proven Anti-Inflammatory Agent, in the Treatment of Ischemic Retinopathies? Antioxidants 2019, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Pizzio, G.A. Potential Implications of the Phytohormone Abscisic Acid in Human Health Improvement at the Central Nervous System. Ann. Epidemiol. Public Health 2022, 5, 1090. [Google Scholar]
- Kim, S.; Hong, I.; Woo, S.; Jang, H.; Pak, S.; Han, S. Isolation of Abscisic Acid from Korean Acacia Honey with Anti-Helicobacter pylori Activity. Pharmacogn. Mag. 2017, 13, S170–S173. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N.S. Antiviral Roles of Abscisic Acid in Plants. Front. Plant Sci. 2017, 8, 1760. [Google Scholar] [CrossRef] [PubMed]
- Bridi, R.; Echeverria, J.; Larena, A.; Nunez Pizarro, P.; Atala, E.; De Camargo, A.C.; Oh, W.Y.; Shahidi, F.; Garcia, O.; Ah-Hen, K.S.; et al. Honeybee Pollen From Southern Chile: Phenolic Profile, Antioxidant Capacity, Bioaccessibility, and Inhibition of DNA Damage. Front. Pharmacol. 2022, 13, 775219. [Google Scholar] [CrossRef] [PubMed]
- Habryka, C.; Socha, R.; Juszczak, L. The Influence of Bee Bread on Antioxidant Properties, Sensory and Quality Characteristics of Multifloral Honey. Appl. Sci. 2023, 13, 7913. [Google Scholar] [CrossRef]
- Aylanc, V.; Tomas, A.; Russo-Almeida, P.; Falcao, S.I.; Vilas-Boas, M. Assessment of Bioactive Compounds under Simulated Gastrointestinal Digestion of Bee Pollen and Bee Bread: Bioaccessibility and Antioxidant Activity. Antioxidants 2021, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Martau, G.A.; Bernadette-Emoke, T.; Odocheanu, R.; Soporan, D.A.; Bochis, M.; Simon, E.; Vodnar, D.C. Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules 2023, 28, 1533. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef] [PubMed]
- Bakour, M.; Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Ousaaid, D.; Teixeira, J.A.; Lyoussi, B. Exploring the Palynological, Chemical, and Bioactive Properties of Non-Studied Bee Pollen and Honey from Morocco. Molecules 2022, 27, 5777. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to Gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Lobiuc, A.; Paval, N.E.; Mangalagiu, I.I.; Gheorghita, R.; Teliban, G.C.; Amariucai-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential Use of Phenolic Acids as Anti-Candida Agents: A Review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Qian, W.; Yang, M.; Wang, T.; Sun, Z.; Liu, M.; Zhang, J.; Zeng, Q.; Cai, C.; Li, Y. Antibacterial Mechanism of Vanillic Acid on Physiological, Morphological, and Biofilm Properties of Carbapenem-Resistant Enterobacter hormaechei. J. Food Prot. 2020, 83, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Akhir, R.A.M.; Abu Bakar, M.F.; Sanusi, S.B. Antioxidant and Antimicrobial Activity of Stingless Bee Bread and Propolis Extracts. AIP Conf. Proc. 2017, 1891, 020090. [Google Scholar] [CrossRef]
- Elsayed, N.; El-Din, H.S.; Altemimi, A.B.; Ahmed, H.Y.; Pratap-Singh, A.; Abedelmaksoud, T.G. In Vitro Antimicrobial, Antioxidant and Anticancer Activities of Egyptian Citrus Beebread. Molecules 2021, 26, 2433. [Google Scholar] [CrossRef] [PubMed]
- Kirci, D.; Demirci, F.; Demirci, B. Microbial Transformation of Hesperidin and Biological Evaluation. ACS Omega 2023, 8, 42610–42621. [Google Scholar] [CrossRef] [PubMed]
- Hudz, N.; Yezerska, O.; Grygorieva, O.; Felsöciová, S.; Brindza, J.; Wieczorek, P.P.; Kacániová, M. Analytical Procedure Elaboration of Total Flavonoid Content Determination and Antimicrobial Activity of Bee Bread Extracts. Acta Pol. Pharm. 2019, 76, 439–452. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, S.H.; Lee, K.A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Abouda, Z.; Zerdani, I.; Kalalou, I.; Faid, M.; Ahami, M.T. The Antibacterial Activity of Maroccan Bee Bread and Bee-Pollen (Fresh and Dried) against Pathogenic Bacteria. Res. J. Microbiol. 2011, 6, 376–384. [Google Scholar]
- Urcan, A.; Criste, A.; Dezmirean, D.; Bobiș, O.; Mărghitaș, L.; Mărgăoan, R.; Hrinca, A. Antimicrobial Activity of Bee Bread Extracts Against Different Bacterial Strains. Bull. UASVM Anim. Sci. Biotechnol. 2018, 75, 85–91. [Google Scholar] [CrossRef]
- Jiang, L.; Li, H.; Wang, L.; Song, Z.; Shi, L.; Li, W.; Deng, X.; Wang, J. Isorhamnetin Attenuates Staphylococcus aureus-Induced Lung Cell Injury by Inhibiting Alpha-Hemolysin Expression. J. Microbiol. Biotechnol. 2016, 26, 596–602. [Google Scholar] [CrossRef]
- Gutierrez-Venegas, G.; Gomez-Mora, J.A.; Meraz-Rodriguez, M.A.; Flores-Sanchez, M.A.; Ortiz-Miranda, L.F. Effect of flavonoids on antimicrobial activity of microorganisms present in dental plaque. Heliyon 2019, 5, e03013. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X.; et al. Kaempferol Inhibits the Primary Attachment Phase of Biofilm Formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef] [PubMed]
- Şeker, M.E.; Ay, E.; KaraÇelİk, A.A.; HÜseyİnoĞlu, R.; Efe, D. First determination of some phenolic compounds and antimicrobial activities of Geranium ibericum subsp. jubatum: A plant endemic to Turkey. Turk. J. Chem. 2021, 45, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Li, A.P.; He, Y.H.; Zhang, S.Y.; Shi, Y.P. Antibacterial activity and action mechanism of flavonoids against phytopathogenic bacteria. Pestic. Biochem. Physiol. 2022, 188, 105221. [Google Scholar] [CrossRef]
- Atsalakis, E.; Chinou, I.; Makropoulou, M.; Karabournioti, S.; Graikou, K. Evaluation of Phenolic Compounds in Cistus creticus Bee Pollen from Greece. Antioxidant and Antimicrobial Properties. Nat. Prod. Commun. 2017, 12, 1934578X1701201141. [Google Scholar]
- Rojo, S.; Escuredo, O.; Rodriguez-Flores, M.S.; Seijo, M.C. Botanical Origin of Galician Bee Pollen (Northwest Spain) for the Characterization of Phenolic Content and Antioxidant Activity. Foods 2023, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Marinas, I.C.; Oprea, E.; Gaboreanu, D.M.; Gradisteanu Pircalabioru, G.; Buleandra, M.; Nagoda, E.; Badea, I.A.; Chifiriuc, M.C. Chemical and Biological Studies of Achillea setacea Herba Essential Oil-First Report on Some Antimicrobial and Antipathogenic Features. Antibiotics 2023, 12, 371. [Google Scholar] [CrossRef]
- Babota, M.; Mocan, A.; Vlase, L.; Crisan, O.; Ielciu, I.; Gheldiu, A.M.; Vodnar, D.C.; Crisan, G.; Paltinean, R. Phytochemical Analysis, Antioxidant and Antimicrobial Activities of Helichrysum arenarium (L.) Moench. and Antennaria dioica (L.) Gaertn. Flowers. Molecules 2018, 23, 409. [Google Scholar] [CrossRef]
- Pelka, K.; Otlowska, O.; Worobo, R.W.; Szweda, P. Bee Bread Exhibits Higher Antimicrobial Potential Compared to Bee Pollen. Antibiotics 2021, 10, 125. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Adaškevičiūtė, V.; Kaškonas, P.; Mickienė, R.; Maruška, A. Antimicrobial and antioxidant activities of natural and fermented bee pollen. Food Biosci. 2020, 34, 100532. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]








| Sample | TPC (GAE) 1 | TFC (QE) 2 | TEAC 3 |
|---|---|---|---|
| BBE1 | 12.40 ± 0.010 | 0.87 ± 0.502 | 0.07 ± 0.020 |
| BBE2 | 7.10 ± 0.005 | 0.52 ± 0.031 | 0.02 ± 0.010 |
| BBE3 | 12.50 ± 0.005 | 1.86 ± 0.516 | 0.03 ± 0.005 |
| BBE4 | 14.40 ± 0.010 | 0.53 ± 0.020 | 0.04 ± 0.006 |
| BBE5 | 11.40 ± 0.005 | 0.50 ± 0.052 | 0.04 ± 0.009 |
| BBE6 | 13.60 ± 0.005 | 0.60 ± 0.051 | 0.06 ± 0.010 |
| BBE7 | 11.20 ± 0.025 | 0.45 ± 0.035 | 0.04 ± 0.004 |
| BBE8 | 18.30 ± 0.029 | 0.52 ± 0.090 | 0.05 ± 0.020 |
| BBE9 | 15.80 ± 0.047 | 0.59 ± 0.030 | 0.05 ± 0.008 |
| BBE10 | 18.30 ± 0.051 | 0.70 ± 0.050 | 0.05 ± 0.050 |
| BBE11 | 14.90 ± 0.017 | 0.95 ± 0.011 | 0.03 ± 0.030 |
| BBE12 | 11.20 ± 0.015 | 0.85 ± 0.500 | 0.02 ± 0.005 |
| Phenolic Compound | Sample | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BB1 | BB2 | BB3 | BB4 | BB5 | BB6 | BB7 | BB8 | BB9 | BB10 | BB11 | BB12 | |
| Phenolic acids | ||||||||||||
| cinnamic acid | 10.65 ± 0.13 | 64.67 ± 0.30 | 10.43 ± 0.14 | 8.65 ± 0.33 | 10.39 ± 0.21 | 15.98 ± 0.49 | 6.63 ± 0.28 | 7.65 ± 0.26 | 11.85 ± 0.34 | 9.91 ± 0.23 | 22.41 ± 0.12 | 12.58 ± 0.22 |
| p-coumaric acid | 58.02 ± 0.21 | 66.50 ± 0.52 | 67.10 ± 0.18 | 67.32 ± 0.78 | 73.29 ± 0.07 | 84.19 ± 0.61 | 79.09 ± 0.48 | 75.85 ± 0.51 | 109.13 ± 0.45 | 64.35 ± 0.12 | 69.43 ± 0.13 | 98.71 ± 0.18 |
| ferulic acid | 7.00 ± 0.14 | 27.20 ± 0.62 | 7.69 ± 0.27 | 29.27 ± 0.07 | 24.06 ± 0.40 | 14.85 ± 0.11 | 15.05 ± 0.40 | 14.38 ± 0.31 | 34.10 ± 0.36 | 16.94 ± 0.09 | 8.43 ± 0.06 | 41.46 ± 0.11 |
| caffeic acid | 52.27 ± 0.20 | 76.76 ± 0.95 | 54.41 ± 0.28 | 66.73 ± 0.98 | 74.48 ± 0.27 | 54.12 ± 0.62 | 74.66 ± 0.27 | 64.20 ± 0.43 | 84.85 ± 0.38 | 53.29 ± 0.43 | 55.28 ± 0.54 | 76.90 ± 0.36 |
| chlorogenic acid | 38.86 ± 0.54 | 40.60 ± 0.25 | 54.10 ± 0.06 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 53.94 ± 0.31 | N.D. |
| 4-hidroxybenzoic acid | 50.22 ± 0.47 | 47.73 ± 0.42 | 49.20 ± 0.11 | 47.32 ± 0.79 | 45.94 ± 0.74 | 46.29 ± 0.38 | 44.84 ± 0.88 | 46.73 ± 0.37 | 47.65 ± 0.24 | 47.59 ± 0.32 | 46.90 ± 0.35 | 47.37 ± 0.46 |
| vanillic acid | 30.84 ± 0.44 | 31.88 ± 0.16 | 39.39 ± 0.38 | 30.17 ± 0.40 | 34.73 ± 0.23 | 30.70 ± 0.40 | 30.64 ± 0.13 | 31.14 ± 0.69 | 30.66 ± 0.56 | 30.97 ± 0.14 | 29.27 ± 0.14 | 34.71 ± 0.27 |
| syringic acid | 30.32 ± 0.27 | 30.72 ± 0.35 | 30.19 ± 0.42 | 30.77 ± 0.76 | 30.84 ± 0.85 | 30.84 ± 0.17 | 30.73 ± 0.10 | 30.49 ± 0.38 | 30.30 ± 0.27 | 30.04 ± 0.06 | 30.07 ± 0.09 | 30.57 ± 0.09 |
| gallic acid | 58.02 ± 0.65 | 66.50 ± 0.40 | 67.10 ± 0.11 | 67.32 ± 0.66 | 73.29 ± 0.10 | 84.19 ± 0.35 | 79.09 ± 0.62 | 75.85 ± 0.30 | 109.13 ± 0.56 | 64.35 ± 0.26 | 69.43 ± 0.43 | 98.71 ± 0.45 |
| Flavonoids | ||||||||||||
| catechin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 36.01 ± 0.58 | 34.54 ± 0.34 | 34.62 ± 0.17 | N.D. | N.D. |
| epicatechin | 51.48 ± 0.21 | N.D. | 51.25 ± 0.59 | 51.28 ± 0.35 | 51.23 ± 0.17 | 51.24 ± 0.16 | N.D. | 51.27 ± 0.20 | 51.28 ± 0.55 | 51.24 ± 0.24 | 51.33 ± 0.44 | 51.23 ± 0.56 |
| myricetin | N.D. | 45.07 ± 0.34 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| quercetin | 1001.36 ± 1.57 | 1178.45 ± 1.00 | 1012.16 ± 0.55 | 1014.32 ± 0.57 | 1182.06 ± 0.74 | 1188.93 ± 0.64 | 1160.44 ± 0.69 | 1121.08 ± 0.57 | 1192.04 ± 0.84 | 1007.52 ± 0.65 | 993.36 ± 0.32 | 1023.75 ± 1.02 |
| apigenin | 30.88 ± 0.41 | 322.38 ± 0.59 | 42.20 ± 0.16 | 57.28 ± 0.46 | 36.88 ± 0.16 | 36.98 ± 0.52 | 35.85 ± 0.42 | 44.48 ± 0.34 | 47.45 ± 0.54 | 49.86 ± 0.28 | 32.15 ± 0.22 | 88.94 ± 0.24 |
| galangin | 7.58 ± 0.14 | 341.12 ± 0.45 | 20.66 ± 0.89 | 37.88 ± 0.62 | 14.58 ± 0.42 | 14.69 ± 0.30 | 13.38 ± 0.04 | 23.24 ± 0.34 | 26.66 ± 0.23 | 29.41 ± 0.14 | 9.16 ± 0.32 | 74.14 ± 0.45 |
| kaempferol | 816.86 ± 1.37 | 984.68 ± 1.17 | 1266.22 ± 0.64 | 831.16 ± 0.55 | 1449.53 ± 0.44 | 1513.51 ± 0.57 | 1354.52 ± 0.65 | 1655.62 ± 0.92 | 1892.27 ± 1.17 | 810.46 ± 0.67 | 762.24 ± 0.76 | 943.44 ± 0.33 |
| isorhamnetin | 466.10 ± 1.44 | 671.82 ± 1.40 | 530.69 ± 0.37 | 587.13 ± 0.69 | 523.09 ± 0.37 | 523.95 ± 0.95 | 532.70 ± 0.74 | 543.26 ± 0.76 | 540.78 ± 0.87 | 464.43 ± 0.34 | 485.41 ± 0.59 | 1333.09 ± 0.60 |
| chrysin | 65.95 ± 0.45 | 65.59 ± 0.23 | 63.09 ± 0.44 | 82.79 ± 0.27 | 63.57 ± 0.21 | 66.43 ± 0.17 | 64.35 ± 0.42 | 62.65 ± 0.24 | 62.28 ± 0.32 | 69.76 ± 0.26 | 68.83 ± 23 | 69.54 ± 0.54 |
| pinocembrin | 52.65 ± 0.61 | 52.66 ± 0.45 | 50.18 ± 0.20 | 67.87 ± 0.54 | 52.39 ± 0.33 | 53.60 ± 0.10 | 52.28 ± 0.45 | 50.96 ± 0.74 | 50.68 ± 0.26 | 54.55 ± 0.34 | 55.19 ± 0.40 | 52.57 ± 0.21 |
| pinostrombin | 64.70 ± 0.40 | 64.60 ± 0.57 | 64.40 ± 0.38 | 65.23 ± 0.58 | 65.78 ± 0.30 | 64.27 ± 0.14 | 67.17 ± 0.57 | 64.43 ± 0.38 | 64.50 ± 0.37 | 64.83 ± 0.78 | 65.01 ± 0.72 | 67.70 ± 0.75 |
| Heteroside Flavonoids | ||||||||||||
| rutin | 66.76 ± 0.89 | 332.03 ± 0.35 | 84.19 ± 0.20 | 113.74 ± 0.30 | 61.22 ± 0.16 | 91.78 ± 0.20 | 56.51 ± 0.52 | 72.50 ± 0.55 | 76.89 ± 0.45 | 99.65 ± 0.65 | 76.27 ± 43 | 383.49 ± 0.57 |
| hesperidin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 52.17 ± 0.21 | N.D. | N.D. | N.D. | N.D. |
| ∑ phenolic acids, flavonoids, and heterosides | 2960.54 | 4510.98 | 3564.65 | 3256.25 | 3867.34 | 3966.54 | 3697.90 | 4123.97 | 4497.02 | 3053.76 | 2984.11 | 4528.91 |
| Stilbenoide | ||||||||||||
| resveratrol | 77.54 ± 0.61 | 79.51 ± 0.51 | 77.29 ± 0.42 | 76.77 ± 0.39 | 76.82 ± 0.35 | 77.41 ± 0.28 | 76.80 ± 0.78 | 76.86 ± 0.34 | 76.41 ± 0.45 | 76.45 ± 0.14 | 76.54 ± 0.54 | 76.54 ± 0.13 |
| Sesquiterpene | ||||||||||||
| abscisic acid | 783.39 ± 0.54 | 4619.24 ± 0.85 | 2344.76 ± 0.75 | 379.04 ± 0.48 | 352.79 ± 0.85 | 718.90 ± 0.42 | 2088.84 ± 1.26 | 2241.88 ± 1.33 | 14,316.31 ± 0.78 | 547.64 ± 0.62 | 190.51 ± 0.38 | 276.63 ± 0.40 |
| Total | 3821.47 | 9209.73 | 5986.70 | 3712.07 | 4296.95 | 4762.84 | 5863.54 | 6442.72 | 18,889.74 | 3677.84 | 3251.17 | 4882.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilie, C.-I.; Spoiala, A.; Geana, E.-I.; Chircov, C.; Ficai, A.; Ditu, L.-M.; Oprea, E. Bee Bread: A Promising Source of Bioactive Compounds with Antioxidant Properties—First Report on Some Antimicrobial Features. Antioxidants 2024, 13, 353. https://doi.org/10.3390/antiox13030353
Ilie C-I, Spoiala A, Geana E-I, Chircov C, Ficai A, Ditu L-M, Oprea E. Bee Bread: A Promising Source of Bioactive Compounds with Antioxidant Properties—First Report on Some Antimicrobial Features. Antioxidants. 2024; 13(3):353. https://doi.org/10.3390/antiox13030353
Chicago/Turabian StyleIlie, Cornelia-Ioana, Angela Spoiala, Elisabeta-Irina Geana, Cristina Chircov, Anton Ficai, Lia-Mara Ditu, and Eliza Oprea. 2024. "Bee Bread: A Promising Source of Bioactive Compounds with Antioxidant Properties—First Report on Some Antimicrobial Features" Antioxidants 13, no. 3: 353. https://doi.org/10.3390/antiox13030353
APA StyleIlie, C. -I., Spoiala, A., Geana, E. -I., Chircov, C., Ficai, A., Ditu, L. -M., & Oprea, E. (2024). Bee Bread: A Promising Source of Bioactive Compounds with Antioxidant Properties—First Report on Some Antimicrobial Features. Antioxidants, 13(3), 353. https://doi.org/10.3390/antiox13030353